BiovaxID (dasiprotimut-T)
Unlike a preventative vaccine, such as for measles or mumps, BiovaxID is administered as a therapeutic cancer vaccine, designed to stimulate and "train" the patient's immune system to respond and attack cancerous cells, even long after therapy has been stopped — each vaccine being unique to that particular patient. Beginning with an excisional (>2 cm) lymph node biopsy, tumor cells are fused with Biovest's proprietary mouse/human heterohybridoma in order to induce secretion of normally surface-bound tumor immunoglobulin (idiotype or Id). Id-secreting clones are identified by a bioinformatics approach which confirms a match of each vaccine's unique idiotype sequence to the tumor's after which they are cultured (expanded) in a personal-scale, disposable hollow-fiber AutovaxID™ bioreactor system. In this manner, each vaccine is highly-specifically matched to highly-unique segments of the patient's tumor genome. During culture, supernatant (containing idiotype) is collected until sufficient amounts have been produced to yield adequate dosage of vaccine. This supernatant is purified by affinity chromatography and conjugated (bonded) to keyhole limpet haemocyanin (KLH), an immune-stimulating carrier protein, resulting in a finished vaccine that can be shipped and administered to patients. In the Phase III clinical trial, manufacturing success was approximately 95% of treated patients. [4]
The BiovaxID vaccine is manufactured through a process known as rescue fusion hybridization. [5] Since BiovaxID is a personalized vaccine, each patient's vaccine is individually manufactured from a tissue biopsy obtained from a patient's own tumor. This approach is used because there is a unique protein called an “idiotype” expressed exclusively on the cancerous B-cells. So, when a full, high-fidelity copy of the idiotype is linked to a foreign protein (KLH), and administered with an immune-enhancing agent (GM-CSF), the resulting vaccine can mount a highly specific anti-lymphoma attack that “trains” the body's own immune system to solely recognize the idiotype as a “foreign invader”, thus stimulating and recruiting the patient's own immune system to destroy micro-pockets of cancer cells that may remain following chemotherapy and potentially target and destroy newly arising lymphoma cells, thus delaying or preventing cancer recurrence. As such, through its unique mode of action, and exemplary safety record, BiovaxID represents a new therapeutic approach to treating follicular lymphoma. [4]
BiovaxID obtained Orphan drug status with the FDA and has obtained positive Phase III clinical trial results. [6] After a median follow-up of 4.71 years (56.6 months, range: 12.6 - 89.3 months), the median disease-free survival in the BiovaxID arm was 44.2 months compared with 30.6 months in the control arm, which is a clinically and statistically significant difference (adj. p=0.029). [7] Among the 75 patients receiving BiovaxID in the study, 35 received BiovaxID manufactured with an IgM isotype and 40 received BiovaxID manufactured with an IgG isotype with each treatment vaccine produced to correspond with the patient's tumor immunoglobulin isotype. Of 40 patients receiving control, 25 had tumors with IgM isotype and 15 had tumors with IgG isotype. Two of the patients in the vaccinated treatment/control population had a tumor with mixed IgM/IgG isotypes and were excluded from this analysis. Among 35 patients with IgM tumor isotype receiving BiovaxID manufactured with an IgM isotype, median time to relapse after randomization was 52.9 months versus 28.7 months in the IgM tumor isotype control-treated patients (log-rank p=0.001; HR=0.34 (p=0.002); [95% CI: 0.17-0.68]. Among 40 patients with IgG tumor isotype receiving BiovaxiD manufactured with an IgG isotype, median time to relapse after randomization was 35.1 months, versus 32.4 months in control-treated patients with IgG tumor isotype (log-rank p=0.807; HR=1.1 (p=0.807): [95% CI: 0.50-2.44]. [8]
In its multi-center, randomized, controlled Phase 3 clinical study, BiovaxID demonstrated that it can induce powerful anti-tumor immune responses while providing a median disease-free survival benefit of over 15 months, and a reduction of 42% in the risk of relapse, and in the company's Phase 2 clinical trial, 28% of patients who received BiovaxID remain in continuous remission at a median of 12.7 years of follow-up. [9]
The approval of Dendreon's Provenge on April 29, 2010, indicates the FDA's willingness to accept a new class of drugs called cancer vaccines, of which BiovaxID is one. BiovaxID has also completed phase II trials for Mantle Cell Lymphoma and is being investigated for the treatment of other Non-Hodgkin's Lymphomas as well. On November 2, 2011, BiovaxID was granted seven years of U.S. market exclusivity for Waldenstrom's Macroglobulinemia, a third and rare type of Non-Hodgkin's Lymphoma. [10]

Lymphoma is a group of blood and lymph tumors that develop from lymphocytes. The name typically refers to just the cancerous versions rather than all such tumours. Signs and symptoms may include enlarged lymph nodes, fever, drenching sweats, unintended weight loss, itching, and constantly feeling tired. The enlarged lymph nodes are usually painless. The sweats are most common at night.

Tumors of the hematopoietic and lymphoid tissues or tumours of the haematopoietic and lymphoid tissues are tumors that affect the blood, bone marrow, lymph, and lymphatic system. Because these tissues are all intimately connected through both the circulatory system and the immune system, a disease affecting one will often affect the others as well, making aplasia, myeloproliferation and lymphoproliferation closely related and often overlapping problems. While uncommon in solid tumors, chromosomal translocations are a common cause of these diseases. This commonly leads to a different approach in diagnosis and treatment of hematological malignancies. Hematological malignancies are malignant neoplasms ("cancer"), and they are generally treated by specialists in hematology and/or oncology. In some centers "hematology/oncology" is a single subspecialty of internal medicine while in others they are considered separate divisions. Not all hematological disorders are malignant ("cancerous"); these other blood conditions may also be managed by a hematologist.
A cancer vaccine, or oncovaccine, is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.
This is a list of terms related to oncology. The original source for this list was the US National Cancer Institute's public domain Dictionary of Cancer Terms.

Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.
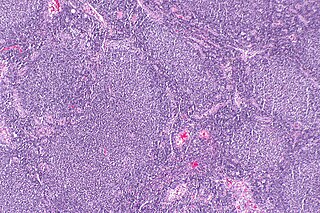
Follicular lymphoma (FL) is a cancer that involves certain types of white blood cells known as lymphocytes. The cancer originates from the uncontrolled division of specific types of B-cells known as centrocytes and centroblasts. These cells normally occupy the follicles in the germinal centers of lymphoid tissues such as lymph nodes. The cancerous cells in FL typically form follicular or follicle-like structures in the tissues they invade. These structures are usually the dominant histological feature of this cancer.
Tositumomab is a murine monoclonal antibody which targets the CD20 antigen produced in mammalian cell. It was combined with iodine-131 to produce a radiopharmaceutical for unsealed source radiotherapy, Iodine-131 Tositumomab, for the treatment of non-Hodgkins lymphoma. It is classified as a IgG2a lambda antibody.

Monoclonal antibodies (mAbs) have varied therapeutic uses. It is possible to create a mAb that binds specifically to almost any extracellular target, such as cell surface proteins and cytokines. They can be used to render their target ineffective, to induce a specific cell signal, to cause the immune system to attack specific cells, or to bring a drug to a specific cell type.
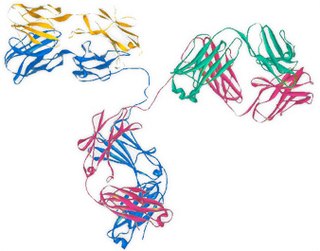
Abagovomab is a mouse anti-idiotype monoclonal antibody whose variable epitope mirrors a tumour antigen (CA-125) highly expressed in the epithelial ovarian cancer. Abagovomab does not bind directly to CA-125, but it works as a "surrogate" antigen, enabling the immune system to identify and attack tumour cells displaying the CA-125 protein. Through this, it is hoped that the body's immune system may be able to combat any remaining individual tumour cells and thus prevent recurrence of the disease.
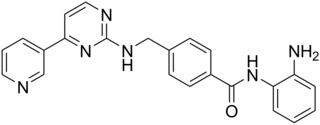
Mocetinostat (MGCD0103) is a benzamide histone deacetylase inhibitor undergoing clinical trials for treatment of various cancers including follicular lymphoma, Hodgkin's lymphoma and acute myelogenous leukemia.
Brentuximab vedotin, sold under the brand name Adcetris, is an antibody-drug conjugate medication used to treat relapsed or refractory Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (ALCL), a type of T cell non-Hodgkin lymphoma. It selectively targets tumor cells expressing the CD30 antigen, a defining marker of Hodgkin lymphoma and ALCL. The drug is being jointly marketed by Millennium Pharmaceuticals outside the US and by Seagen in the US.

Phosphoinositide 3-kinase inhibitors are a class of medical drugs that are mainly used to treat advanced cancers. They function by inhibiting one or more of the phosphoinositide 3-kinase (PI3K) enzymes, which are part of the PI3K/AKT/mTOR pathway. This signal pathway regulates cellular functions such as growth and survival. It is strictly regulated in healthy cells, but is always active in many cancer cells, allowing the cancer cells to better survive and multiply. PI3K inhibitors block the PI3K/AKT/mTOR pathway and thus slow down cancer growth. They are examples of a targeted therapy. While PI3K inhibitors are an effective treatment, they can have very severe side effects and are therefore only used if other treatments have failed or are not suitable.

Avax Technologies, Inc is a Philadelphia-based biotechnology company whose most advanced product candidate is MVax for melanoma. MVax is a cancer vaccine that received a Special Protocol Assessment agreement with the FDA in October 2006, and subsequently began a Phase III registration clinical trial in November 2007. In previous studies, MVax demonstrated a 5-year overall survival rate (OS)of 44% and response rate of 35%.

Tower Cancer Research Foundation (TCRF) is a 501(c)(3) non-profit organization dedicated to clinical research, patient support and community education. It was established in 1996 in Beverly Hills, California.
Rescue fusion hybridization is a process used to manufacture some therapeutic cancer vaccines in which individual tumor cells obtained through biopsy are fused with an antibody-secreting cell to form a heterohybridoma. This cell then secretes the unique idiotype, or immunoglobulin antigen characteristic of the individual tumor, which is purified for use as the vaccine. It is used to produce the BiovaxID vaccine for follicular lymphoma.
Racotumomab is a therapeutic cancer vaccine for the treatment of solid tumors that is currently under clinical development by Recombio, an international public-private consortium with the participation of the Center of Molecular Immunology at Havana, Cuba (CIM) and researchers from Buenos Aires University and National University of Quilmes in Argentina. It induces the patient's immune system to generate a response against a cancer-specific molecular target with the purpose of blocking tumor growth, slowing disease progression and ultimately increasing patient survival.
Kite Pharma is an American biotechnology company that develops cancer immunotherapy products, with a primary focus on genetically engineered autologous CAR T cell therapy, a cell-based therapy which relies on chimeric antigen receptors and T cells. Founded in 2009, and based in Santa Monica, California, it was acquired by Gilead Sciences in 2017.
PROSTVAC is a cancer immunotherapy candidate in clinical development by Bavarian Nordic for the treatment of all prostate cancer although clinical trials are focusing on more advanced cases of metastatic castration-resistant prostate cancer (mCRPC). PROSTVAC is a vaccine designed to enable the immune system to recognize and attack prostate cancer cells by triggering a specific and targeted T cell immune response to cancer cells that express the tumor-associated antigen prostate-specific antigen (PSA).

Copanlisib, sold under the brand name Aliqopa, is a medication used for the treatment of adults experiencing relapsed follicular lymphoma who have received at least two prior systemic therapies.
The Immune Response Corporation (IRC) was a pharmaceutical company that worked in the development immunotherapeutic products. The firm was founded by Jonas Salk and Kevin Kimberlin when Kimberlin, "asked Salk to become lead scientific advisor for a new biotech company specializing in 'anti-idiotypes,' a novel vaccine technology." Salk called the proposal "liberating."









