
Biogen Inc. is an American multinational biotechnology company based in Cambridge, Massachusetts, United States specializing in the discovery, development, and delivery of therapies for the treatment of neurological diseases to patients worldwide. Biogen operates in Argentina, Brazil, Canada, China, France, Germany, Hungary, India, Italy, Japan, Mexico, Netherlands, Poland, Sweden, and Switzerland.
Antisense therapy is a form of treatment that uses antisense oligonucleotides (ASOs) to target messenger RNA (mRNA). ASOs are capable of altering mRNA expression through a variety of mechanisms, including ribonuclease H mediated decay of the pre-mRNA, direct steric blockage, and exon content modulation through splicing site binding on pre-mRNA. Several ASOs have been approved in the United States, the European Union, and elsewhere.

Cabergoline, sold under the brand name Dostinex among others, is a dopaminergic medication used in the treatment of high prolactin levels, prolactinomas, Parkinson's disease, and for other indications. It is taken by mouth.

4-Aminopyridine (4-AP) is an organic compound with the chemical formula H2NC5H4N. It is one of the three isomeric aminopyridines. It is used as a research tool in characterizing subtypes of the potassium channel. It has also been used as a drug, to manage some of the symptoms of multiple sclerosis, and is indicated for symptomatic improvement of walking in adults with several variations of the disease. It was undergoing Phase III clinical trials as of 2008, and the U.S. Food and Drug Administration (FDA) approved the compound on January 22, 2010. Fampridine is also marketed as Ampyra in the United States by Acorda Therapeutics and as Fampyra in the European Union, Canada, and Australia. In Canada, the medication has been approved for use by Health Canada since February 10, 2012.
Ipratropium bromide/salbutamol, sold under the brand name Combivent among others, is a combination medication used to treat chronic obstructive pulmonary disease (COPD). It contains ipratropium and salbutamol.

Entacapone, sold under the brand name Comtan among others, is a medication commonly used in combination with other medications for the treatment of Parkinson's disease. Entacapone together with levodopa and carbidopa allows levodopa to have a longer effect in the brain and reduces Parkinson's disease signs and symptoms for a greater length of time than levodopa and carbidopa therapy alone.
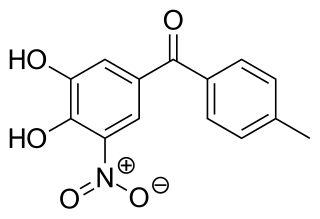
Tolcapone, sold under the brand name Tasmar, is a medication used to treat Parkinson's disease (PD). It is a selective, potent and reversible nitrocatechol-type inhibitor of the enzyme catechol-O-methyltransferase (COMT). It has demonstrated significant liver toxicity, which has led to suspension of marketing authorisations in a number of countries.
Inhalable insulin is a powdered form of insulin, delivered with an inhaler into the lungs where it is absorbed. In general, inhaled insulins have been more rapidly absorbed than subcutaneous injected insulin, with faster peak concentration in serum and more rapid metabolism.

Merz Pharma GmbH & Co. KGaA is an internationally active family-owned company, headquartered in Frankfurt am Main, Germany. Merz is the parent company of independent businesses in the fields of aesthetic medicine, therapeutic medicine, and wellness and beauty products with its brands Tetesept and Merz Spezial.
Cephalon, Inc. was an American biopharmaceutical company co-founded in 1987 by pharmacologist Frank Baldino, Jr., neuroscientist Michael Lewis, and organic chemist James C. Kauer—all three former scientists with the DuPont Company. Baldino served as Cephalon's chairman and chief executive officer, until his death in December 2010. The company's name comes from the adjective "cephalic" meaning "related to the head or brain", as it was established primarily to pursue treatments for neurodegenerative diseases.
Ocrelizumab, sold under the brand name Ocrevus, is a medication used for the treatment of multiple sclerosis. It is a humanized anti-CD20 monoclonal antibody. It targets CD20 marker on B lymphocytes and is an immunosuppressive drug. Ocrelizumab binds to an epitope that overlaps with the epitope to which rituximab binds. It is administered by intravenous infusion. The fixed-dose combination ocrelizumab/hyaluronidase is administered by subcutaneous injection.

Biotie Therapies Oyj was a Finnish biotechnology and pharmaceutics company that was acquired by Acorda Therapeutics in January 2016. The company's research and development was focused on drugs for neurodegenerative and psychiatric disorders like Parkinson's disease, Alzheimer's disease and other cognitive disorders, alcohol and drug dependence and post traumatic stress disorder, and inflammatory and fibrotic liver disease. The company's headquarters is in Turku, Western Finland, and it is listed on NASDAQ OMX Helsinki.
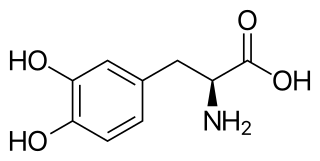
Levodopa, also known as L-DOPA and sold under many brand names, is a dopaminergic medication which is used in the treatment of Parkinson's disease and certain other conditions like dopamine-responsive dystonia and restless legs syndrome. The drug is usually used and formulated in combination with a peripherally selective aromatic L-amino acid decarboxylase (AAAD) inhibitor like carbidopa or benserazide. Levodopa is taken by mouth, by inhalation, through an intestinal tube, or by administration into fat.

Remacemide is a drug which acts as a low-affinity NMDA antagonist with sodium channel blocking properties. It has been studied for the treatment of acute ischemic stroke, epilepsy, Huntington's disease, and Parkinson's disease.
Carbidopa/levodopa/entacapone, sold under the brand name Stalevo among others, is a dopaminergic fixed-dose combination medication that contains carbidopa, levodopa, and entacapone for the treatment of Parkinson's disease.

Safinamide, sold under the brand name Xadago, is a medication used as treatment for Parkinson's disease with "off" episodes; it has multiple modes of action, including the inhibition of monoamine oxidase B.

Teriflunomide, sold under the brand name Aubagio, is the active metabolite of leflunomide. Teriflunomide was investigated in the Phase III clinical trial TEMSO as a medication for multiple sclerosis (MS). The study was completed in July 2010. 2-year results were positive. However, the subsequent TENERE head-to-head comparison trial reported that "although permanent discontinuations [of therapy] were substantially less common among MS patients who received teriflunomide compared with interferon beta-1a, relapses were more common with teriflunomide." The drug was approved for use in the United States in September 2012 and for use in the European Union in August 2013.

Pimavanserin, sold under the brand name Nuplazid, is an atypical antipsychotic which is approved for the treatment of Parkinson's disease psychosis. Unlike other antipsychotics, pimavanserin is not a dopamine receptor antagonist, but rather is a selective inverse agonist of the serotonin 5-HT2A receptor.
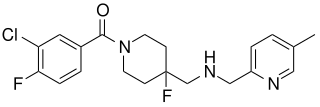
Befiradol is an experimental drug being studied for the treatment of levodopa-induced dyskinesia. It is a potent and selective 5-HT1A receptor full agonist.
XP-21279 is a sustained-release levodopa (L-DOPA) prodrug and hence a dopamine precursor and non-selective dopamine receptor agonist which was under development for the treatment of Parkinson's disease. It is taken by mouth.














