
A monoclonal antibody is an antibody produced from a cell lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell.
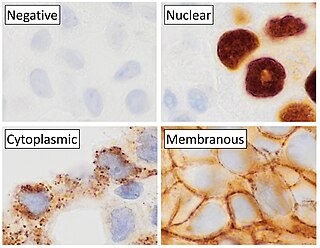
Immunohistochemistry (IHC) is the most common application of immunostaining. It involves the process of selectively identifying antigens (proteins) in cells of a tissue section by exploiting the principle of antibodies binding specifically to antigens in biological tissues. IHC takes its name from the roots "immuno", in reference to antibodies used in the procedure, and "histo", meaning tissue. Albert Coons conceptualized and first implemented the procedure in 1941.

Rituximab, sold under the brand name Rituxan among others, is a monoclonal antibody medication used to treat certain autoimmune diseases and types of cancer. It is used for non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, granulomatosis with polyangiitis, idiopathic thrombocytopenic purpura, pemphigus vulgaris, myasthenia gravis and Epstein–Barr virus-positive mucocutaneous ulcers. It is given by slow intravenous infusion. Biosimilars of Rituxan include Blitzima, Riabni, Ritemvia, Rituenza, Rixathon, Ruxience, and Truxima.
In biology, chimeric antigen receptors (CARs)—also known as chimeric immunoreceptors, chimeric T cell receptors or artificial T cell receptors—are receptor proteins that have been engineered to give T cells the new ability to target a specific antigen. The receptors are chimeric in that they combine both antigen-binding and T cell activating functions into a single receptor.

Cancer immunotherapy is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.

In immunology, epitope mapping is the process of experimentally identifying the binding site, or epitope, of an antibody on its target antigen. Identification and characterization of antibody binding sites aid in the discovery and development of new therapeutics, vaccines, and diagnostics. Epitope characterization can also help elucidate the binding mechanism of an antibody and can strengthen intellectual property (patent) protection. Experimental epitope mapping data can be incorporated into robust algorithms to facilitate in silico prediction of B-cell epitopes based on sequence and/or structural data.

A single-domain antibody (sdAb), also known as a NANOBODY®, is an antibody fragment consisting of a single monomeric variable antibody domain. Like a whole antibody, it is able to bind selectively to a specific antigen. With a molecular weight of only 12–15 kDa, single-domain antibodies are much smaller than common antibodies which are composed of two heavy protein chains and two light chains, and even smaller than Fab fragments and single-chain variable fragments.

B-lymphocyte antigen CD20 or CD20 is expressed on the surface of all B-cells beginning at the pro-B phase and progressively increasing in concentration until maturity.

Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAbs) to bind monospecifically to certain cells or proteins. The objective is that this treatment will stimulate the patient's immune system to attack those cells. Alternatively, in radioimmunotherapy a radioactive dose localizes a target cell line, delivering lethal chemical doses. Antibodies are used to bind to molecules involved in T-cell regulation to remove inhibitory pathways that block T-cell responses. This is known as immune checkpoint therapy.
Fusion proteins or chimeric (kī-ˈmir-ik) proteins are proteins created through the joining of two or more genes that originally coded for separate proteins. Translation of this fusion gene results in a single or multiple polypeptides with functional properties derived from each of the original proteins. Recombinant fusion proteins are created artificially by recombinant DNA technology for use in biological research or therapeutics. Chimeric or chimera usually designate hybrid proteins made of polypeptides having different functions or physico-chemical patterns. Chimeric mutant proteins occur naturally when a complex mutation, such as a chromosomal translocation, tandem duplication, or retrotransposition creates a novel coding sequence containing parts of the coding sequences from two different genes. Naturally occurring fusion proteins are commonly found in cancer cells, where they may function as oncoproteins. The bcr-abl fusion protein is a well-known example of an oncogenic fusion protein, and is considered to be the primary oncogenic driver of chronic myelogenous leukemia.
Small modular immunopharmaceuticals, or SMIPs for short, are artificial proteins that are intended for use as pharmaceutical drugs. They are largely built from parts of antibodies (immunoglobulins), and like them have a binding site for antigens that could be used for monoclonal antibody therapy. SMIPs have similar biological half-life and, being smaller than antibodies, are reasoned to have better tissue penetration properties. They were invented by Trubion and are now being developed by Emergent BioSolutions, which acquired Trubion in 2010.
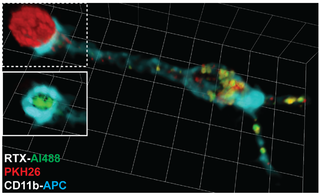
Trogocytosis is when a cell nibbles another cell. It is a process whereby lymphocytes conjugated to antigen-presenting cells extract surface molecules from these cells and express them on their own surface. The molecular reorganization occurring at the interface between the lymphocyte and the antigen-presenting cell during conjugation is also called "immunological synapse".

Nventa Biopharmaceuticals Corporation was a Canadian-incorporated biopharmaceutical company headquartered in San Diego, California developing therapeutics for the treatment of viral infections and cancer, focusing on diseases caused by human papillomavirus (HPV). Nventa is currently the only company applying heat shock protein (Hsp) technology to target the over 20 million Americans already infected with HPV. Previously headquartered in Victoria, British Columbia, Canada, the company’s common stock traded on the Toronto Stock Exchange under the symbol: NVN.
CancerVax was an American pharmaceutical company founded in 1998 by Donald Morton. The company sought to develop a vaccine for cancer, and had candidates for melanoma reach phase III clinical trials. When those trials proved unsuccessful in 2005, the company soon underwent a reverse takeover with Micromet.
Nuvelo Inc. was a biopharmaceutical company engaged in the discovery, development and commercialization of drugs for acute cardiovascular disease, cancer and other debilitating medical conditions. On January 27, 2009, the company was acquired by ARCA Biopharma, Inc. in a reverse takeover transaction.
DARPins are genetically engineered antibody mimetic proteins typically exhibiting highly specific and high-affinity target protein binding. They are derived from natural ankyrin repeat proteins, one of the most common classes of binding proteins in nature, which are responsible for diverse functions such as cell signaling, regulation and structural integrity of the cell. DARPins consist of at least three, repeat motifs or modules, of which the most N- and the most C-terminal modules are referred to as "caps", since they shield the hydrophobic core of the protein. The number of internal modules is indicated as number while the caps are indicated with "N" or "C", respectively. The molecular mass of e.g. 14 or 18 kDa (kilodaltons) for four- (N2C) or five- (N3C) repeat DARPins is rather small for a biologic.
Gene expression profiling has revealed that diffuse large B-cell lymphoma (DLBCL) is composed of at least 3 different sub-groups, each having distinct oncogenic mechanisms that respond to therapies in different ways. Germinal Center B-Cell like (GCB) DLBCLs appear to arise from normal germinal center B cells, while Activated B-cell like (ABC) DLBCLs are thought to arise from postgerminal center B cells that are arrested during plasmacytic differentiation. The differences in gene expression between GCB DLBCL and ABC DLBCL are as vast as the differences between distinct types of leukemia, but these conditions have historically been grouped together and treated as the same disease.
Regulus Therapeutics Inc. or Regulus is a clinical stage biopharmaceutical company focused on the development of first-in-class drugs that target microRNAs to treat a broad range of diseases. Regulus was established in September 2007 by Alnylam Pharmaceuticals and Isis Pharmaceuticals.
Adenovirus varieties have been explored extensively as a viral vector for gene therapy and also as an oncolytic virus.
Arcturus Therapeutics is an American RNA medicines biotechnology company focused on the discovery, development and commercialization of therapeutics for rare diseases and infectious diseases. Arcturus has developed a novel, potent, and safe RNA therapeutics platform called Lunar, a proprietary lipid-enabled delivery system for nucleic acid medicines including small interfering RNA (siRNA), messenger RNA (mRNA), gene editing RNA, DNA, antisense oligonucleotides (ASO), and microRNA.











