
A retrovirus is a type of virus that inserts a DNA copy of its RNA genome into the DNA of a host cell that it invades, thus changing the genome of that cell. After invading a host cell's cytoplasm, the virus uses its own reverse transcriptase enzyme to produce DNA from its RNA genome, the reverse of the usual pattern, thus retro (backwards). The new DNA is then incorporated into the host cell genome by an integrase enzyme, at which point the retroviral DNA is referred to as a provirus. The host cell then treats the viral DNA as part of its own genome, transcribing and translating the viral genes along with the cell's own genes, producing the proteins required to assemble new copies of the virus. Many retroviruses cause serious diseases in humans, other mammals, and birds.
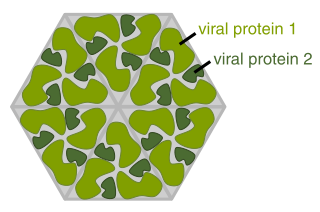
The term viral protein refers to both the products of the genome of a virus and any host proteins incorporated into the viral particle. Viral proteins are grouped according to their functions, and groups of viral proteins include structural proteins, nonstructural proteins, regulatory proteins, and accessory proteins. Viruses are non-living and do not have the means to reproduce on their own, instead depending on their host cell's machinery to do this. Thus, viruses do not code for most of the proteins required for their replication and the translation of their mRNA into viral proteins, but use proteins encoded by the host cell for this purpose.
Metaviridae is a family of viruses which exist as Ty3-gypsy LTR retrotransposons in a eukaryotic host's genome. They are closely related to retroviruses: members of the family Metaviridae share many genomic elements with retroviruses, including length, organization, and genes themselves. This includes genes that encode reverse transcriptase, integrase, and capsid proteins. The reverse transcriptase and integrase proteins are needed for the retrotransposon activity of the virus. In some cases, virus-like particles can be formed from capsid proteins.
Lentivirus is a genus of retroviruses that cause chronic and deadly diseases characterized by long incubation periods, in humans and other mammalian species. The genus includes the human immunodeficiency virus (HIV), which causes AIDS. Lentiviruses are distributed worldwide, and are known to be hosted in apes, cows, goats, horses, cats, and sheep as well as several other mammals.

Gammaretrovirus is a genus in the Retroviridae family. Example species are the murine leukemia virus and the feline leukemia virus. They cause various sarcomas, leukemias and immune deficiencies in mammals, reptiles and birds.
The genome and proteins of HIV (human immunodeficiency virus) have been the subject of extensive research since the discovery of the virus in 1983. "In the search for the causative agent, it was initially believed that the virus was a form of the Human T-cell leukemia virus (HTLV), which was known at the time to affect the human immune system and cause certain leukemias. However, researchers at the Pasteur Institute in Paris isolated a previously unknown and genetically distinct retrovirus in patients with AIDS which was later named HIV." Each virion comprises a viral envelope and associated matrix enclosing a capsid, which itself encloses two copies of the single-stranded RNA genome and several enzymes. The discovery of the virus itself occurred two years following the report of the first major cases of AIDS-associated illnesses.
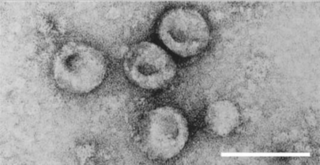
Pestivirus is a genus of viruses, in the family Flaviviridae. Viruses in the genus Pestivirus infect mammals, including members of the family Bovidae and the family Suidae. There are 11 species in this genus. Diseases associated with this genus include: hemorrhagic syndromes, abortion, and fatal mucosal disease.
The murine leukemia viruses are retroviruses named for their ability to cause cancer in murine (mouse) hosts. Some MLVs may infect other vertebrates. MLVs include both exogenous and endogenous viruses. Replicating MLVs have a positive sense, single-stranded RNA (ssRNA) genome that replicates through a DNA intermediate via the process of reverse transcription.
Human foamy virus (HFV) is a retrovirus in the genus Spumavirus. The spumaviruses are complex and significantly different from the other six genera of retroviruses in several ways. The foamy viruses derive their name from the characteristic ‘foamy’ appearance of the cytopathic effect (CPE) induced in the cells. Foamy virus in humans occurs only as a result of zoonotic infection.

A viral envelope is the outermost layer of many types of viruses. It protects the genetic material in their life cycle when traveling between host cells. Not all viruses have envelopes. A viral envelope protein or E protein is a protein in the envelope, which may be acquired by the capsid from an infected host cell.
Simian foamy virus (SFV) is a species of the genus Spumavirus that belongs to the family of Retroviridae. It has been identified in a wide variety of primates, including prosimians, New World and Old World monkeys, as well as apes, and each species has been shown to harbor a unique (species-specific) strain of SFV, including African green monkeys, baboons, macaques, and chimpanzees. As it is related to the more well-known retrovirus human immunodeficiency virus (HIV), its discovery in primates has led to some speculation that HIV may have been spread to the human species in Africa through contact with blood from apes, monkeys, and other primates, most likely through bushmeat-hunting practices.

Molluscum contagiosum virus (MCV) is a species of DNA poxvirus that causes the human skin infection molluscum contagiosum. Molluscum contagiosum affects about 200,000 people a year, about 1% of all diagnosed skin diseases. Diagnosis is based on the size and shape of the skin lesions and can be confirmed with a biopsy, as the virus cannot be routinely cultured. Molluscum contagiosum virus is the only species in the genus Molluscipoxvirus. MCV is a member of the subfamily Chordopoxvirinae of family Poxviridae. Other commonly known viruses that reside in the subfamily Chordopoxvirinae are variola virus and monkeypox virus.
Bovine immunodeficiency virus (BIV) is a retrovirus belonging to the genus Lentivirus. It is similar to the human immunodeficiency virus (HIV) and infects cattle. The cells primarily infected are lymphocytes and monocytes/macrophages.
Mason-Pfizer monkey virus (M-PMV), formerly Simian retrovirus (SRV), is a species of retroviruses that usually infect and cause a fatal immune deficiency in Asian macaques. The ssRNA virus appears sporadically in mammary carcinoma of captive macaques at breeding facilities which expected as the natural host, but the prevalence of this virus in feral macaques remains unknown. M-PMV was transmitted naturally by virus-containing body fluids, via biting, scratching, grooming, and fighting. Cross contaminated instruments or equipment (fomite) can also spread this virus among animals.
Entomopoxvirinae is a subfamily of viruses, in the family Poxviridae. Insects, human, vertebrates, and arthropods serve as natural hosts. There are currently 31 species in this subfamily, divided among 4 genera with one species unassigned to a genus. Diseases associated with this subfamily include: impairment of motility and development.
Feline foamy virus or Feline syncytial virus is a retrovirus and belongs to the family Retroviridae and the subfamily Spumaretrovirinae. It shares the genus Felispumavirus with only Puma feline foamy virus. There has been controversy on whether FeFV is nonpathogenic as the virus is generally asymptomatic in affected cats and does not cause disease. However, some changes in kidney and lung tissue have been observed over time in cats affected with FeFV, which may or may not be directly affiliated. This virus is fairly common and infection rates gradually increase with a cat's age. Study results from antibody examinations and PCR analysis have shown that over 70% of felines over 9 years old were seropositive for Feline foamy virus. Viral infections are similar between male and female domesticated cats whereas in the wild, more feral females cats are affected with FeFV.
Equine foamy virus (EFV), also called foamy virus (FV), is virus in the genus Equispumavirus. It shares similarities, with respect to replication, with lentiviruses. EFV, along with other FVs are from the family Retroviridae and subfamily Spumaretrovirinae. Spumarivuses, such as EFV, are complicated retroviruses that have been characterized in many animals including nonhuman primates, cattle, cats. Additionally, these viruses have been identified in animals that most often carry lentiviruses.

Ground squirrel hepatitis virus, abbreviated GSHV, is a partially double-stranded DNA virus that is closely related to human Hepatitis B virus (HBV) and Woodchuck hepatitis virus (WHV). It is a member of the family of viruses Hepadnaviridae and the genus Orthohepadnavirus. Like the other members of its family, GSHV has high degree of species and tissue specificity. It was discovered in Beechey ground squirrels, Spermophilus beecheyi, but also infects Arctic ground squirrels, Spermophilus parryi. Commonalities between GSHV and HBV include morphology, DNA polymerase activity in genome repair, cross-reacting viral antigens, and the resulting persistent infection with viral antigen in the blood (antigenemia). As a result, GSHV is used as an experimental model for HBV.
Sepik virus (SEPV) is an arthropod-borne virus (arbovirus) of the genus Flavivirus and family Flaviviridae. Flaviviridae is one of the most well characterized viral families, as it contains many well-known viruses that cause diseases that have become very prevalent in the world, like Dengue virus. The genus Flavivirus is one of the largest viral genera and encompasses over 50 viral species, including tick and mosquito borne viruses like Yellow fever virus and West Nile virus. Sepik virus is much less well known and has not been as well-classified as other viruses because it has not been known of for very long. Sepik virus was first isolated in 1966 from the mosquito Mansoniaseptempunctata, and it derives its name from the Sepik River area in Papua New Guinea, where it was first found. The geographic range of Sepik virus is limited to Papua New Guinea, due to its isolation.

Modoc virus (MODV) is a rodent-associated flavivirus. Small and enveloped, MODV contains positive single-stranded RNA. Taxonomically, MODV is part of the Flavivirus genus and Flaviviridae family. The Flavivirus genus includes nearly 80 viruses, both vector-borne and no known vector (NKV) species. Known flavivirus vector-borne viruses include Dengue virus, Yellow Fever virus, tick-borne encephalitis virus, and West Nile virus.










