Valine (symbol Val or V) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isopropyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, beans and legumes. It is encoded by all codons starting with GU (GUU, GUC, GUA, and GUG).
In molecular biology, protein catabolism is the breakdown of proteins into smaller peptides and ultimately into amino acids. Protein catabolism is a key function of digestion process. Protein catabolism often begins with pepsin, which converts proteins into polypeptides. These polypeptides are then further degraded. In humans, the pancreatic proteases include trypsin, chymotrypsin, and other enzymes. In the intestine, the small peptides are broken down into amino acids that can be absorbed into the bloodstream. These absorbed amino acids can then undergo amino acid catabolism, where they are utilized as an energy source or as precursors to new proteins.
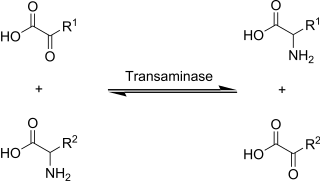
Transamination is a chemical reaction that transfers an amino group to a ketoacid to form new amino acids. This pathway is responsible for the deamination of most amino acids. This is one of the major degradation pathways which convert essential amino acids to non-essential amino acids.

Aspartate transaminase (AST) or aspartate aminotransferase, also known as AspAT/ASAT/AAT or (serum) glutamic oxaloacetic transaminase, is a pyridoxal phosphate (PLP)-dependent transaminase enzyme that was first described by Arthur Karmen and colleagues in 1954. AST catalyzes the reversible transfer of an α-amino group between aspartate and glutamate and, as such, is an important enzyme in amino acid metabolism. AST is found in the liver, heart, skeletal muscle, kidneys, brain, red blood cells and gall bladder. Serum AST level, serum ALT level, and their ratio are commonly measured clinically as biomarkers for liver health. The tests are part of blood panels.

Transaminases or aminotransferases are enzymes that catalyze a transamination reaction between an amino acid and an α-keto acid. They are important in the synthesis of amino acids, which form proteins.
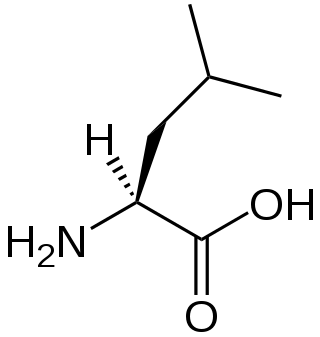
A branched-chain amino acid (BCAA) is an amino acid having an aliphatic side-chain with a branch. Among the proteinogenic amino acids, there are three BCAAs: leucine, isoleucine, and valine. Non-proteinogenic BCAAs include 2-aminoisobutyric acid.

The Cahill cycle, also known as the alanine cycle or glucose-alanine cycle, is the series of reactions in which amino groups and carbons from muscle are transported to the liver. It is quite similar to the Cori cycle in the cycling of nutrients between skeletal muscle and the liver. When muscles degrade amino acids for energy needs, the resulting nitrogen is transaminated to pyruvate to form alanine. This is performed by the enzyme alanine transaminase (ALT), which converts L-glutamate and pyruvate into α-ketoglutarate and L-alanine. The resulting L-alanine is shuttled to the liver where the nitrogen enters the urea cycle and the pyruvate is used to make glucose.

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).
Branched-chain amino acid aminotransferase (BCAT), also known as branched-chain amino acid transaminase, is an aminotransferase enzyme (EC 2.6.1.42) which acts upon branched-chain amino acids (BCAAs). It is encoded by the BCAT2 gene in humans. The BCAT enzyme catalyzes the conversion of BCAAs and α-ketoglutarate into branched chain α-keto acids and glutamate.

Tyrosine aminotransferase is an enzyme present in the liver and catalyzes the conversion of tyrosine to 4-hydroxyphenylpyruvate.

In enzymology, an omega-amidase (EC 3.5.1.3) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2,5-diaminovalerate transaminase is an enzyme that catalyzes the chemical reaction

In enzymology, 4-aminobutyrate transaminase, also called GABA transaminase or 4-aminobutyrate aminotransferase, or GABA-T, is an enzyme that catalyzes the chemical reaction:
In enzymology, a D-amino-acid transaminase is an enzyme that catalyzes the chemical reaction:
In enzymology, glutamate-prephenate aminotransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a leucine transaminase is an enzyme that catalyzes the chemical reaction

Aspartate aminotransferase, mitochondrial is an enzyme that in humans is encoded by the GOT2 gene. Glutamic-oxaloacetic transaminase is a pyridoxal phosphate-dependent enzyme which exists in cytoplasmic and inner-membrane mitochondrial forms, GOT1 and GOT2, respectively. GOT plays a role in amino acid metabolism and the urea and Kreb's cycle. Also, GOT2 is a major participant in the malate-aspartate shuttle, which is a passage from the cytosol to the mitochondria. The two enzymes are homodimeric and show close homology. GOT2 has been seen to have a role in cell proliferation, especially in terms of tumor growth.
Glutaminolysis (glutamine + -lysis) is a series of biochemical reactions by which the amino acid glutamine is lysed to glutamate, aspartate, CO2, pyruvate, lactate, alanine and citrate.

Branched chain ketoacid dehydrogenase kinase (BCKDK) is an enzyme encoded by the BCKDK gene on chromosome 16. This enzyme is part of the mitochondrial protein kinases family and it is a regulator of the valine, leucine, and isoleucine catabolic pathways. BCKDK is found in the mitochondrial matrix and the prevalence of it depends on the type of cell. Liver cells tend to have the lowest concentration of BCKDK, whereas skeletal muscle cells have the highest amount. Abnormal activity of this enzyme often leads to diseases such as maple syrup urine disease and cachexia.

Glutamic--pyruvic transaminase 2 is a protein that in humans is encoded by the GPT2 gene.














