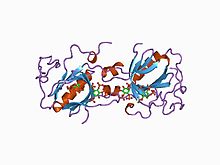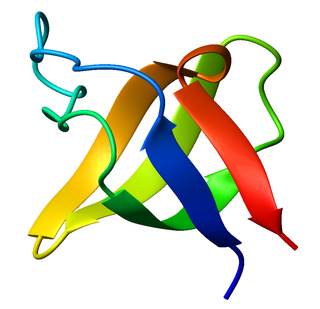
The SRC Homology 3 Domain is a small protein domain of about 60 amino acid residues. Initially, SH3 was described as a conserved sequence in the viral adaptor protein v-Crk. This domain is also present in the molecules of phospholipase and several cytoplasmic tyrosine kinases such as Abl and Src. It has also been identified in several other protein families such as: PI3 Kinase, Ras GTPase-activating protein, CDC24 and cdc25. SH3 domains are found in proteins of signaling pathways regulating the cytoskeleton, the Ras protein, and the Src kinase and many others. The SH3 proteins interact with adaptor proteins and tyrosine kinases. Interacting with tyrosine kinases, SH3 proteins usually bind far away from the active site. Approximately 300 SH3 domains are found in proteins encoded in the human genome. In addition to that, the SH3 domain was responsible for controlling protein-protein interactions in the signal transduction pathways and regulating the interactions of proteins involved in the cytoplasmic signaling.

Histone acetyltransferases (HATs) are enzymes that acetylate conserved lysine amino acids on histone proteins by transferring an acetyl group from acetyl-CoA to form ε-N-acetyllysine. DNA is wrapped around histones, and, by transferring an acetyl group to the histones, genes can be turned on and off. In general, histone acetylation increases gene expression.
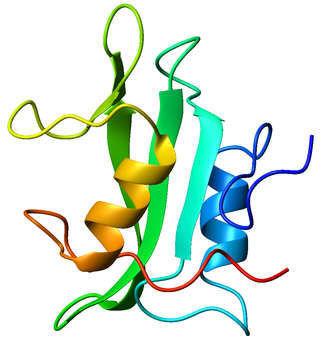
The SH2domain is a structurally conserved protein domain contained within the Src oncoprotein and in many other intracellular signal-transducing proteins. SH2 domains bind to phosphorylated tyrosine residues on other proteins, modifying the function or activity of the SH2-containing protein. The SH2 domain may be considered the prototypical modular protein-protein interaction domain, allowing the transmission of signals controlling a variety of cellular functions. SH2 domains are especially common in adaptor proteins that aid in the signal transduction of receptor tyrosine kinase pathways.

Pleckstrin homology domain or (PHIP) is a protein domain of approximately 120 amino acids that occurs in a wide range of proteins involved in intracellular signaling or as constituents of the cytoskeleton.

RAF proto-oncogene serine/threonine-protein kinase, also known as proto-oncogene c-RAF or simply c-Raf or even Raf-1, is an enzyme that in humans is encoded by the RAF1 gene. The c-Raf protein is part of the ERK1/2 pathway as a MAP kinase (MAP3K) that functions downstream of the Ras subfamily of membrane associated GTPases. C-Raf is a member of the Raf kinase family of serine/threonine-specific protein kinases, from the TKL (Tyrosine-kinase-like) group of kinases.

Bruton's tyrosine kinase, also known as tyrosine-protein kinase BTK, is a tyrosine kinase that is encoded by the BTK gene in humans. BTK plays a crucial role in B cell development.
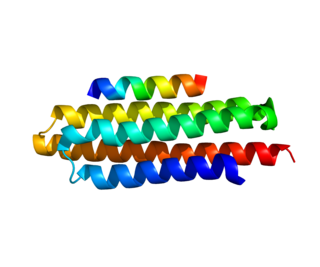
Paxillin is a protein that in humans is encoded by the PXN gene. Paxillin is expressed at focal adhesions of non-striated cells and at costameres of striated muscle cells, and it functions to adhere cells to the extracellular matrix. Mutations in PXN as well as abnormal expression of paxillin protein has been implicated in the progression of various cancers.

Tyrosine-protein kinase ITK/TSK also known as interleukin-2-inducible T-cell kinase or simply ITK, is a protein that in humans is encoded by the ITK gene. ITK is a member of the TEC family of kinases and is highly expressed in T cells.

Frizzled is a family of atypical G protein-coupled receptors that serve as receptors in the Wnt signaling pathway and other signaling pathways. When activated, Frizzled leads to activation of Dishevelled in the cytosol.

GRB2-associated-binding protein 2 also known as GAB2 is a protein that in humans is encoded by the GAB2 gene.

FYVE, RhoGEF and PH domain-containing protein 1 (FGD1) also known as faciogenital dysplasia 1 protein (FGDY), zinc finger FYVE domain-containing protein 3 (ZFYVE3), or Rho/Rac guanine nucleotide exchange factor FGD1 is a protein that in humans is encoded by the FGD1 gene that lies on the X chromosome. Orthologs of the FGD1 gene are found in dog, cow, mouse, rat, and zebrafish, and also budding yeast and C. elegans. It is a member of the FYVE, RhoGEF and PH domain containing family.
Tyrosine-protein kinase Tec is a tyrosine kinase that in humans is encoded by the TEC gene. Tec kinase is expressed in hematopoietic, liver, and kidney cells and plays an important role in T-helper cell processes. Tec kinase is the name-giving member of the Tec kinase family, a family of non-receptor protein-tyrosine kinases.

B-cell linker (BLNK) protein is expressed in B cells and macrophages and plays a large role in B cell receptor signaling. Like all adaptor proteins, BLNK has no known intrinsic enzymatic activity. Its function is to temporally and spatially coordinate and regulate downstream signaling effectors in B cell receptor (BCR) signaling, which is important in B cell development. Binding of these downstream effectors is dependent on BLNK phosphorylation. BLNK is encoded by the BLNK gene and is also known as SLP-65, BASH, and BCA.

Cytoplasmic tyrosine-protein kinase BMX is an enzyme that in humans is encoded by the BMX gene.
A non-receptor tyrosine kinase (nRTK) is a cytosolic enzyme that is responsible for catalysing the transfer of a phosphate group from a nucleoside triphosphate donor, such as ATP, to tyrosine residues in proteins. Non-receptor tyrosine kinases are a subgroup of protein family tyrosine kinases, enzymes that can transfer the phosphate group from ATP to a tyrosine residue of a protein (phosphorylation). These enzymes regulate many cellular functions by switching on or switching off other enzymes in a cell.
Epstein–Barr virus (EBV) latent membrane protein 2 (LMP2) are two viral proteins of the Epstein–Barr virus. LMP2A/LMP2B are transmembrane proteins that act to block tyrosine kinase signaling. LMP2A is a transmembrane protein that inhibits normal B-cell signal transduction by mimicking an activated B-cell receptor (BCR). The N-terminus domain of LMP2A is tyrosine phosphorylated and associates with Src family protein tyrosine kinases (PTKs) as well as spleen tyrosine kinase (Syk). PTKs and Syk are associated with BCR signal transduction.
Dual-specificity phosphatase is a form of phosphatase that can act upon tyrosine or serine/threonine residues.

Zinc finger CCHC-type containing 18 (ZCCHC18) is a protein that in humans is encoded by ZCCHC18 gene. It is also known as Smad-interacting zinc finger protein 2 (SIZN2), para-neoplastic Ma antigen family member 7b (PNMA7B), and LOC644353. Other names such as zinc finger, CCHC domain containing 12 pseudogene 1, P0CG32, ZCC18_HUMAN had been used to describe this protein.

Rubicon is a protein that in humans is encoded by the RUBCN gene. Rubicon is one of the few known negative regulators of autophagy, a cellular process that degrades unnecessary or damaged cellular components. Rubicon is recruited to its sites of action through interaction with the small GTPase Rab7, and impairs the autophagosome-lysosome fusion step of autophagy through inhibition of PI3KC3-C2.

The Rubicon homology domain is an evolutionarily conserved protein domain of approximately 250 amino acids that mediates protein–protein interaction. RH domains are present in several human proteins involved in regulation of autophagy and endosomal trafficking. While not all RH domains have been characterized, those of human Rubicon and PLEKHM1 mediate interaction with the small GTPase Rab7, which is found on late endosomes and autophagosomes.