
Holoprosencephaly (HPE) is a cephalic disorder in which the prosencephalon fails to develop into two hemispheres, typically occurring between the 18th and 28th day of gestation. Normally, the forebrain is formed and the face begins to develop in the fifth and sixth weeks of human pregnancy. The condition also occurs in other species.

Sonic hedgehog protein (SHH) is encoded for by the SHH gene. The protein is named after the video game character Sonic the Hedgehog.

A retinal ganglion cell (RGC) is a type of neuron located near the inner surface of the retina of the eye. It receives visual information from photoreceptors via two intermediate neuron types: bipolar cells and retina amacrine cells. Retina amacrine cells, particularly narrow field cells, are important for creating functional subunits within the ganglion cell layer and making it so that ganglion cells can observe a small dot moving a small distance. Retinal ganglion cells collectively transmit image-forming and non-image forming visual information from the retina in the form of action potential to several regions in the thalamus, hypothalamus, and mesencephalon, or midbrain.
Cell adhesion molecules (CAMs) are a subset of cell surface proteins that are involved in the binding of cells with other cells or with the extracellular matrix (ECM), in a process called cell adhesion. In essence, CAMs help cells stick to each other and to their surroundings. CAMs are crucial components in maintaining tissue structure and function. In fully developed animals, these molecules play an integral role in generating force and movement and consequently ensuring that organs are able to execute their functions normally. In addition to serving as "molecular glue", CAMs play important roles in the cellular mechanisms of growth, contact inhibition, and apoptosis. Aberrant expression of CAMs may result in a wide range of pathologies, ranging from frostbite to cancer.

L1, also known as L1CAM, is a transmembrane protein member of the L1 protein family, encoded by the L1CAM gene. This protein, of 200 to 220 kDa, is a neuronal cell adhesion molecule with a strong implication in cell migration, adhesion, neurite outgrowth, myelination and neuronal differentiation. It also plays a key role in treatment-resistant cancers due to its function. It was first identified in 1984 by M. Schachner who found the protein in post-mitotic mice neurons.
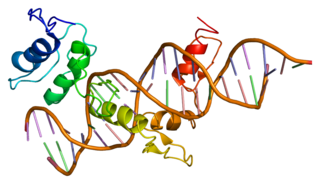
Zinc finger protein GLI1 also known as glioma-associated oncogene is a protein that in humans is encoded by the GLI1 gene. It was originally isolated from human glioblastoma cells.

Zinc finger protein GLI2 also known as GLI family zinc finger 2 is a protein that in humans is encoded by the GLI2 gene. The protein encoded by this gene is a transcription factor.

Zinc finger protein GLI3 is a protein that in humans is encoded by the GLI3 gene.

Cyclopamine (11-deoxojervine) is a naturally occurring steroidal alkaloid. It is a teratogenic component of corn lily, which when consumed during gestation has been demonstrated to induce birth defects, including the development of a single eye (cyclopia) in offspring. The molecule was named after this effect, which was originally observed by Idaho lamb farmers in 1957 after their herds gave birth to cycloptic lambs. It then took more than a decade to identify corn lily as the culprit. Later work suggested that differing rain patterns had changed grazing behaviours, which led to a greater quantity of corn lily to be ingested by pregnant sheep. Cyclopamine interrupts the sonic hedgehog signalling pathway, instrumental in early development, ultimately causing birth defects.
The Hedgehog signaling pathway is a signaling pathway that transmits information to embryonic cells required for proper cell differentiation. Different parts of the embryo have different concentrations of hedgehog signaling proteins. The pathway also has roles in the adult. Diseases associated with the malfunction of this pathway include cancer.

Myogenesis is the formation of skeletal muscular tissue, particularly during embryonic development.

Chemokine ligand 3 (CXCL3) is a small cytokine belonging to the CXC chemokine family that is also known as GRO3 oncogene (GRO3), GRO protein gamma (GROg) and macrophage inflammatory protein-2-beta (MIP2b). CXCL3 controls migration and adhesion of monocytes and mediates its effects on its target cell by interacting with a cell surface chemokine receptor called CXCR2. More recently, it has been shown that Cxcl3 regulates cell autonomously the migration of the precursors of cerebellar granule neurons toward the internal layers of cerebellum, during the morphogenesis of cerebellum. Moreover, if the expression of Cxcl3 is reduced in cerebellar granule neuron precursors, this highly enhances the frequency of the medulloblastoma, the tumor of cerebellum. In fact, the reduced expression of Cxcl3 forces the cerebellar granule neuron precursors to remain at the surface of the cerebellum, where they highly proliferate under the stimulus of Sonic hedgehog, becoming target of transforming insults. Remarkably, the treatment with CXCL3 completely prevents the growth of medulloblastoma lesions in a Shh-type mouse model of medulloblastoma. Thus, CXCL3 is a target for medulloblastoma therapy. Cxcl3 is directly regulated transcriptionally by BTG2

G protein-coupled receptor 56 also known as TM7XN1 is a protein encoded by the ADGRG1 gene. GPR56 is a member of the adhesion GPCR family. Adhesion GPCRs are characterized by an extended extracellular region often possessing N-terminal protein modules that is linked to a TM7 region via a domain known as the GPCR-Autoproteolysis INducing (GAIN) domain.
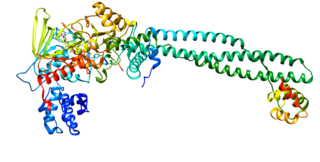
Zinc finger protein SNAI1 is a protein that in humans is encoded by the SNAI1 gene. Snail is a family of transcription factors that promote the repression of the adhesion molecule E-cadherin to regulate epithelial to mesenchymal transition (EMT) during embryonic development.

Indian hedgehog homolog (Drosophila), also known as IHH, is a protein which in humans is encoded by the IHH gene. This cell signaling protein is in the hedgehog signaling pathway. The several mammalian variants of the Drosophila hedgehog gene have been named after the various species of hedgehog; the Indian hedgehog is honored by this one. The gene is not specific to Indian hedgehogs.

Suppressor of fused homolog is a protein that in humans is encoded by the SUFU gene. In molecular biology, the protein domain suppressor of fused protein (Sufu) has an important role in the cell. The Sufu is important in negatively regulating an important signalling pathway in the cell, the Hedgehog signalling pathway (HH). This particular pathway is crucial in embryonic development. There are several homologues of Sufu, found in a wide variety of organisms.

Cadherin-15 is a protein that in humans is encoded by the CDH15 gene.

Brother of CDO is a protein that in humans is encoded by the BOC gene.

Myogenic factor 5 is a protein that in humans is encoded by the MYF5 gene. It is a protein with a key role in regulating muscle differentiation or myogenesis, specifically the development of skeletal muscle. Myf5 belongs to a family of proteins known as myogenic regulatory factors (MRFs). These basic helix loop helix transcription factors act sequentially in myogenic differentiation. MRF family members include Myf5, MyoD (Myf3), myogenin, and MRF4 (Myf6). This transcription factor is the earliest of all MRFs to be expressed in the embryo, where it is only markedly expressed for a few days. It functions during that time to commit myogenic precursor cells to become skeletal muscle. In fact, its expression in proliferating myoblasts has led to its classification as a determination factor. Furthermore, Myf5 is a master regulator of muscle development, possessing the ability to induce a muscle phenotype upon its forced expression in fibroblastic cells.

Myogenic factor 6 is a protein that in humans is encoded by the MYF6 gene. This gene is also known in the biomedical literature as MRF4 and herculin. MYF6 is a myogenic regulatory factor (MRF) involved in the process known as myogenesis.





















