Related Research Articles
The urea cycle (also known as the ornithine cycle) is a cycle of biochemical reactions that produces urea (NH2)2CO from ammonia (NH3). Animals that use this cycle, mainly amphibians and mammals, are called ureotelic.
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid, is the chemical compound with the formula H2NCOOH. It can be obtained by the reaction of ammonia NH3 and carbon dioxide CO2 at very low temperatures, which also yields ammonium carbamate [NH4]+[NH2CO2]−. The compound is stable only up to about 250 K (−23 °C); at higher temperatures it decomposes into those two gases. The solid apparently consists of dimers, with the two molecules connected by hydrogen bonds between the two carboxyl groups –COOH.

Serine dehydratase or L-serine ammonia lyase (SDH) is in the β-family of pyridoxal phosphate-dependent (PLP) enzymes. SDH is found widely in nature, but its structure and properties vary among species. SDH is found in yeast, bacteria, and the cytoplasm of mammalian hepatocytes. SDH catalyzes the deamination of L-serine to yield pyruvate, with the release of ammonia.

The enzyme cystathionine γ-lyase (EC 4.4.1.1, CTH or CSE; also cystathionase; systematic name L-cystathionine cysteine-lyase (deaminating; 2-oxobutanoate-forming)) breaks down cystathionine into cysteine, 2-oxobutanoate (α-ketobutyrate), and ammonia:
The enzyme 3-chloro-D-alanine dehydrochlorinase (EC 4.5.1.2) catalyzes the reaction
The enzyme cysteine-S-conjugate β-lyase (EC 4.4.1.13) catalyzes the chemical reaction
The enzyme diaminopropionate ammonia-lyase (EC 4.3.1.15) catalyzes the chemical reaction
The enzyme D-serine ammonia-lyase (EC 4.3.1.18), with systematic name D-serine ammonia-lyase (pyruvate-forming), catalyzes the chemical reaction
The enzyme Glucosaminate ammonia-lyase (EC 4.3.1.9) catalyzes the chemical reaction
The enzyme homocysteine desulfhydrase (EC 4.4.1.2) catalyzes the chemical reaction
The enzyme L-2-amino-4-chloropent-4-enoate dehydrochlorinase (EC 4.5.1.4) catalyzes the reaction
The enzyme L-cysteate sulfo-lyase (EC 4.4.1.25) catalyzes the reaction
The enzyme L-serine ammonia-lyase (EC 4.3.1.17) catalyzes the chemical reaction

The enzyme methionine γ-lyase (EC 4.4.1.11, MGL) is in the γ-family of PLP-dependent enzymes. It degrades sulfur-containing amino acids to α-keto acids, ammonia, and thiols:
The enzyme Serine-sulfate ammonia-lyase (EC 4.3.1.10) catalyzes the chemical reaction

Threonine ammonia-lyase (EC 4.3.1.19, systematic name L-threonine ammonia-lyase (2-oxobutanoate-forming), also commonly referred to as threonine deaminase or threonine dehydratase, is an enzyme responsible for catalyzing the conversion of L-threonine into α-ketobutyrate and ammonia:
The enzyme 4-(2-carboxyphenyl)-2-oxobut-3-enoate aldolase (EC 4.1.2.34) catalyzes the chemical reaction
In enzymology, a beta-ureidopropionase (EC 3.5.1.6) is an enzyme that catalyzes the chemical reaction
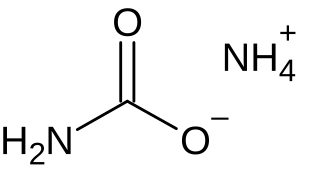
Ammonium carbamate is a chemical compound with the formula [NH4][H2NCO2] consisting of ammonium cation NH+4 and carbamate anion NH2COO−. It is a white solid that is extremely soluble in water, less so in alcohol. Ammonium carbamate can be formed by the reaction of ammonia NH3 with carbon dioxide CO2, and will slowly decompose to those gases at ordinary temperatures and pressures. It is an intermediate in the industrial synthesis of urea (NH2)2CO, an important fertilizer.
Methylenediurea deaminase (EC 3.5.3.21, methylenediurease) is an enzyme with systematic name methylenediurea aminohydrolase found in Brucella anthropi, a bacterium. This enzyme catalyses the following chemical reaction:
References
- Copper AJ, Meister A (1973). "Enzymatic conversion of O-carbamyl-L-serine to pyruvate and ammonia". Biochem. Biophys. Res. Commun. 55 (3): 780–7. doi:10.1016/0006-291X(73)91212-6. PMID 4761084.