Related Research Articles
Carbon compounds are defined as chemical substances containing carbon. More compounds of carbon exist than any other chemical element except for hydrogen. Organic carbon compounds are far more numerous than inorganic carbon compounds. In general bonds of carbon with other elements are covalent bonds. Carbon is tetravalent but carbon free radicals and carbenes occur as short-lived intermediates. Ions of carbon are carbocations and carbanions are also short-lived. An important carbon property is catenation as the ability to form long carbon chains and rings.

Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as halides of carbon without carbon-hydrogen and carbon-carbon bonds, and certain compounds of carbon with nitrogen and oxygen.

A chalcogenide is a chemical compound consisting of at least one chalcogen anion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfides, selenides, tellurides, and polonides, rather than oxides. Many metal ores exist as chalcogenides. Photoconductive chalcogenide glasses are used in xerography. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide MoS2 is a common solid lubricant.
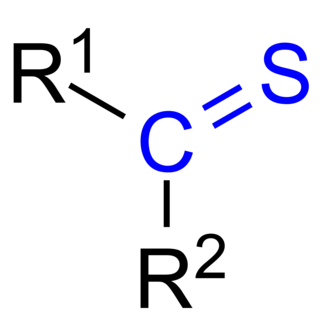
In organic chemistry, thioketones are organosulfur compounds related to conventional ketones in which the oxygen has been replaced by a sulfur. Instead of a structure of R2C=O, thioketones have the structure R2C=S, which is reflected by the prefix "thio-" in the name of the functional group. Thus the simplest thioketone is thioacetone, the sulfur analog of acetone. Unhindered alkylthioketones typically tend to form polymers or rings.
Organoselenium chemistry is the science exploring the properties and reactivity of organoselenium compounds, chemical compounds containing carbon-to-selenium chemical bonds. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.

Thiophosgene is a red liquid with the formula CSCl2. It is a molecule with trigonal planar geometry. There are two reactive C–Cl bonds that allow it to be used in diverse organic syntheses.
Carbonyl fluoride is a chemical compound with the formula COF2. It is a carbon oxohalide. This gas, like its analog phosgene, is colourless and highly toxic. The molecule is planar with C2v symmetry, bond lengths of 1.174 Å (C=O) and 1.312 Å (C–F), and an F–C–F bond angle of 108.0°.

Selenium compounds are compounds containing the element selenium (Se). Among these compounds, selenium has various oxidation states, the most common ones being −2, +4, and +6. Selenium compounds exist in nature in the form of various minerals, such as clausthalite, guanajuatite, tiemannite, crookesite etc., and can also coexist with sulfide minerals such as pyrite and chalcopyrite. For many mammals, selenium compounds are essential. For example, selenomethionine and selenocysteine are selenium-containing amino acids present in the human body. Selenomethionine participates in the synthesis of selenoproteins. The reduction potential and pKa (5.47) of selenocysteine are lower than those of cysteine, making some proteins have antioxidant activity. Selenium compounds have important applications in semiconductors, glass and ceramic industries, medicine, metallurgy and other fields.

Carbon diselenide is an inorganic compound with the chemical formula CSe2. It is a yellow-orange oily liquid with pungent odor. It is the selenium analogue of carbon disulfide and carbon dioxide. This light-sensitive compound is insoluble in water and soluble in organic solvents.

Iodine pentoxide is the chemical compound with the formula I2O5. This iodine oxide is the anhydride of iodic acid, and the only stable oxide of iodine. It is produced by dehydrating iodic acid at 200 °C in a stream of dry air:

Sodium selenide is an inorganic compound of sodium and selenium with the chemical formula Na2Se.
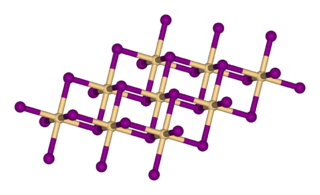
Titanium diselenide (TiSe2) also known as titanium(IV) selenide, is an inorganic compound of titanium and selenium. In this material selenium is viewed as selenide (Se2−) which requires that titanium exists as Ti4+. Titanium diselenide is a member of metal dichalcogenides, compounds that consist of a metal and an element of the chalcogen column within the periodic table. Many exhibit properties of potential value in battery technology, such as intercalation and electrical conductivity, although most applications focus on the less toxic and lighter disulfides, e.g. TiS2.
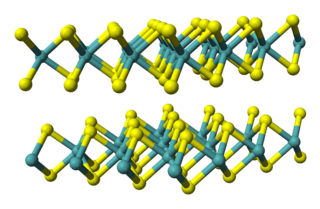
Molybdenum diselenide is an inorganic compound of molybdenum and selenium. Its structure is similar to that of MoS
2. Compounds of this category are known as transition metal dichalcogenides, abbreviated TMDCs. These compounds, as the name suggests, are made up of a transition metals and elements of group 16 on the periodic table of the elements. Compared to MoS
2, MoSe
2 exhibits higher electrical conductivity.
Phosphorus selenides are a relatively obscure group of compounds. There have been some studies of the phosphorus - selenium phase diagram and the glassy amorphous phases are reported. The compounds that have been reported are shown below. While some of phosphorus selenides are similar to their sulfide analogues, there are some new forms, molecular P2Se5 and the polymeric catena-[P4Se4]x. There is also some doubt about the existence of molecular P4Se10.
Hydrogen chalcogenides are binary compounds of hydrogen with chalcogen atoms. Water, the first chemical compound in this series, contains one oxygen atom and two hydrogen atoms, and is the most common compound on the Earth's surface.
Tellurium compounds are compounds containing the element tellurium (Te). Tellurium belongs to the chalcogen family of elements on the periodic table, which also includes oxygen, sulfur, selenium and polonium: Tellurium and selenium compounds are similar. Tellurium exhibits the oxidation states −2, +2, +4 and +6, with +4 being most common.

Niobium diselenide or niobium(IV) selenide is a layered transition metal dichalcogenide with formula NbSe2. Niobium diselenide is a lubricant, and a superconductor at temperatures below 7.2 K that exhibit a charge density wave (CDW). NbSe2 crystallizes in several related forms, and can be mechanically exfoliated into monatomic layers, similar to other transition metal dichalcogenide monolayers. Monolayer NbSe2 exhibits very different properties from the bulk material, such as of Ising superconductivity, quantum metallic state, and strong enhancement of the CDW.

Rhenium diselenide is an inorganic compound with the formula ReSe2. It has a layered structure where atoms are strongly bonded within each layer. The layers are held together by weak Van der Waals bonds, and can be easily peeled off from the bulk material.
Carbon oxohalides are a group of chemical compounds that contain only carbon, oxygen and halogen atoms: fluorine, chlorine, bromine and iodine. They include carbonyl halides, COX2, and oxalyl halides, C2X2O2, where X = F, Cl, Br or I. The halogen atoms X do not have to be identical; they differ in mixed oxohalides. Most combinations of halogens exist but carbonyl iodide, COI2, is unknown. The carbon–oxygen bond length in carbonyl halides (1.13–1.17 Å) is shorter than in other carbonyl compounds such as aldehydes and ketones, carboxylic acids, esters and amides. They are reactive reagents for halogenation, acylation and dehydration reactions.
References
- 1 2 3 4 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 306, 314–319, 754–755. ISBN 978-0-08-037941-8.
- ↑ Housecroft, C. E.; Sharpe, A. G. (2008). Inorganic Chemistry (3rd ed.). Prentice Hall. pp. 409–412, 423–425. ISBN 978-0-13-175553-6.
- 1 2 3 4 5 Wells, A. F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford University Press. p. 926. ISBN 978-0-19-965763-6.
- ↑ Plyler, Earle K.; Blaine, Lamdin R.; Tidwell, Eugene D. (1955). "Infrared absorption and emission spectra of carbon monoxide in the region from 4 to 6 microns". Journal of Research of the National Bureau of Standards. 55 (4): 183–192. doi: 10.6028/jres.055.019 .
- 1 2 3 4 5 6 7 8 William M. Haynes, ed. (2012). CRC Handbook of Chemistry and Physics (93rd ed.). CRC Press. p. 9–33. ISBN 978-1439880500.
- 1 2 Hardy, W. A.; Silvey, G. (1954). "Microwave Spectrum of TeCS and Masses of the Stable Tellurium Isotopes". Phys. Rev. 95 (2): 385–. doi:10.1103/PhysRev.95.385.
- ↑ Powell, B. M.; Torrie, B. H. (1983). "Structure of solid carbon diselenide (CSe2) at 17.5, 50 and 200K". Acta Crystallogr. C . 39 (8): 3070–3072. doi:10.1107/S0108270183007015.