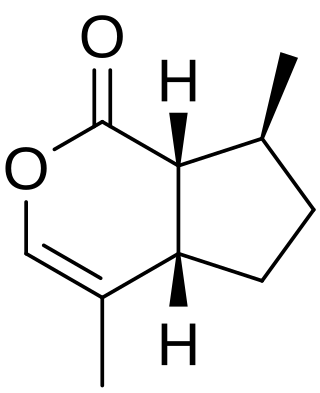
Nepetalactone is a name for multiple iridoid analog stereoisomers. Nepetalactones are produced by Nepeta cataria (catnip) and many other plants belonging to the genus Nepeta, in which they protect these plants from herbivorous insects by functioning as insect repellents. They are also produced by many aphids, in which they are sex pheromones. Nepetalactones are cat attractants, and cause the behavioral effects that catnip induces in domestic cats. However, they affect visibly only about two thirds of adult cats. They produce similar behavioral effects in many other felids, especially in lions and jaguars. In 1941, the research group of Samuel M. McElvain was the first to determine the structures of nepetalactones and several related compounds.
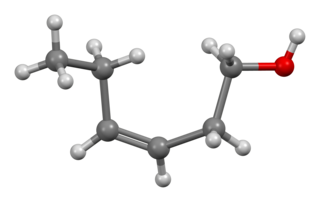
1-Hexanol (IUPAC name hexan-1-ol) is an organic alcohol with a six-carbon chain and a condensed structural formula of CH3(CH2)5OH. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. Two additional straight chain isomers of 1-hexanol, 2-hexanol and 3-hexanol, exist, both of which differing by the location of the hydroxyl group. Many isomeric alcohols have the formula C6H13OH. It is used in the perfume industry.

Chemical ecology is a vast and interdisciplinary field utilizing biochemistry, biology, ecology, and organic chemistry for explaining observed interactions of living things and their environment through chemical compounds. Early examples of the field trace back to experiments with the same plant genus in different environments, interaction of plants and butterflies, and the behavioral effect of catnip. Chemical ecologists seek to identify the specific molecules that function as signals mediating community or ecosystem processes and to understand the evolution of these signals. The chemicals behind such roles are typically small, readily-diffusible organic molecules that act over various distances that are dependent on the environment but can also include larger molecules and small peptides.

An aroma compound, also known as an odorant, aroma, fragrance or flavoring, is a chemical compound that has a smell or odor. For an individual chemical or class of chemical compounds to impart a smell or fragrance, it must be sufficiently volatile for transmission via the air to the olfactory system in the upper part of the nose. As examples, various fragrant fruits have diverse aroma compounds, particularly strawberries which are commercially cultivated to have appealing aromas, and contain several hundred aroma compounds.
cis-3-Hexen-1-ol, also known as (Z)-3-hexen-1-ol and leaf alcohol, is a colorless oily liquid with an intense grassy-green odor of freshly cut green grass and leaves. It is produced in small amounts by most plants and it acts as an attractant to many predatory insects. cis-3-Hexen-1-ol is an important aroma compound that is used in fruit and vegetable flavors and in perfumes. The yearly production is about 30 tonnes. Its esters are also important flavor and fragrance raw materials.

Citral is an acyclic monoterpene aldehyde. Being a monoterpene, it is made of two isoprene units. Citral is a collective term which covers two geometric isomers that have their own separate names; the E-isomer is named geranial or citral A. The Z-isomer is named neral or citral B. These stereoisomers occur as a mixture, often not in equal proportions; e.g. in essential oil of Australian ginger, the neral to geranial ratio is 0.61.
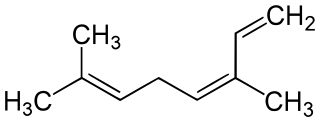
Ocimenes are a group of isomeric hydrocarbons. The ocimenes are monoterpenes found within a variety of plants and fruits. α-Ocimene and the two β-ocimenes differ in the position of the isolated double bond: it is terminal in the alpha isomer. α-Ocimene is cis-3,7-dimethyl-1,3,7-octatriene. β-Ocimene is trans-3,7-dimethyl-1,3,6-octatriene. β-Ocimene exists in two stereoisomeric forms, cis and trans, with respect to the central double bond.
Salicylic aldehyde (2-hydroxybenzaldehyde) is an organic compound with the formula C6H4OH(CHO). Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. This colorless oily liquid has a bitter almond odor at higher concentration. Salicylaldehyde is a precursor to coumarin and a variety of chelating agents.

2-Ethylhexanol is an organic compound with the chemical formula CH3CH2CH2CH2CH(CH2CH3)CH2OH. It is a branched, eight-carbon chiral alcohol. It is a colorless liquid that is poorly soluble in water but soluble in most organic solvents. It is produced on a large scale (>2,000,000,000 kg/y) for use in numerous applications such as solvents, flavors, and fragrances and especially as a precursor for production of other chemicals such as emollients and plasticizers. It is encountered in plants, fruits, and wines. The odor has been reported as "heavy, earthy, and slightly floral" for the R enantiomer and "a light, sweet floral fragrance" for the S enantiomer.

3-Mercapto-3-methylbutan-1-ol, also known as MMB, is a common odorant found in food and cat urine. The aromas ascribed to MMB include catty, roasty, broth-like, meaty, and savory, or similar to cooked leeks.
Heptanal or heptanaldehyde is an alkyl aldehyde. It is a colourless liquid with a strong fruity odor, which is used as precursor to components in perfumes and lubricants.

Phenylacetaldehyde is an organic compound used in the synthesis of fragrances and polymers. Phenylacetaldehyde is an aldehyde that consists of acetaldehyde bearing a phenyl substituent; the parent member of the phenylacetaldehyde class of compounds. It has a role as a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is an alpha-CH2-containing aldehyde and a member of phenylacetaldehydes.

The sense of smell, or olfaction, is the special sense through which smells are perceived. The sense of smell has many functions, including detecting desirable foods, hazards, and pheromones, and plays a role in taste.
Green leaf volatiles (GLV) are organic compounds released by plants. Some of these chemicals function as signaling compounds between either plants of the same species, of other species, or even different lifeforms like insects.

Bisabolenes are a group of closely related natural chemical compounds which are classified as sesquiterpenes. Bisabolenes are produced from farnesyl pyrophosphate (FPP) and are present in the essential oils of bisabol, and of a wide variety of other plants including cubeb, lemon, and oregano. Various derivates also function as pheromones in different insects, such as stink bugs and fruit flies. Bisabolenes are produced by several fungi, though their biological role in that group of organisms remains unclear.
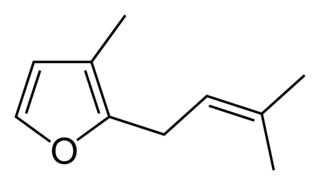
Rosefuran (3-methyl-2-prenylfuran) is an organic compound, classified as a terpenoid. It is a minor constituent of the aroma of the rose. Rosefuran is a 2,3-disubstituted furan. It has an odor threshold of 200 ppb and constitutes 0.16% of Bulgarian rose oil. Rosefuran has been established as a female sex pheromone of an acarid mite, Caloglyphus sp. Concentrations of less than 100 ng of synthetic rosefuran caused sexual excitation in males of the species.

6-Nonenal is an organic compound with the formula C2H5CH=CH(CH2)4CHO. Other isomeric nonenal compounds are also known to exist naturally, e.g. 2-nonenal. The cis-isomer of 6-nonenal is often listed as the principal component in the aromas of muskmelon fruits. The trans-isomer is listed as an off-flavor aroma of milk foams, and thought to be a possible polypropylene odorant.
2-Decenal (dec-2-enal) is an organic compound with the chemical formula of C10H18O. It exists as a pair of cis and trans isomers, (2E)-2-decenal and (2Z)-2-decenal. It is an oily, clear liquid under normal conditions, that may be yellow due to impurities. 2-Decenal is described as having a strong, waxy odor. It is found in animal food (in trace quantities), and is part of the essential oil of coriander. 2-Decenal is also used as a flavoring agent.

The smell of freshly cut grass is an odour caused by green leaf volatiles (GLVs) released when it is damaged. Mechanical damage to grass from activities such as lawnmowing results in the release of cis-3-hexenal and other compounds that contribute to a grassy or "green" smell. cis-3-Hexenal has a low odour detection threshold that humans can perceive at concentrations as low as 0.25 parts per billion.
trans-2-Hexenal is an organic unsaturated aldehyde with a six-carbon chain. This clear, pale yellow liquid has a green, leafy, herbal fruit smell. It occurs naturally in a wide variety of plants, fruits, vegetables, and spices, and appears to be an important mediating and signalling chemical in plant-fungus and plant-insect interactions, such as the symbiosis between acacia ants and Acacias.















