DCI may be an abbreviation for:

Inositol, or more precisely myo-inositol, is a carbocyclic sugar that is abundant in the brain and other mammalian tissues; it mediates cell signal transduction in response to a variety of hormones, neurotransmitters, and growth factors and participates in osmoregulation.
Hyperinsulinemic hypoglycemia describes the condition and effects of low blood glucose caused by excessive insulin. Hypoglycemia due to excess insulin is the most common type of serious hypoglycemia. It can be due to endogenous or injected insulin.

Inositol trisphosphate receptor (InsP3R) is a membrane glycoprotein complex acting as a Ca2+ channel activated by inositol trisphosphate (InsP3). InsP3R is very diverse among organisms, and is necessary for the control of cellular and physiological processes including cell division, cell proliferation, apoptosis, fertilization, development, behavior, learning and memory. Inositol triphosphate receptor represents a dominant second messenger leading to the release of Ca2+ from intracellular store sites. There is strong evidence suggesting that the InsP3R plays an important role in the conversion of external stimuli to intracellular Ca2+ signals characterized by complex patterns relative to both space and time, such as Ca2+ waves and oscillations.
The molecular formula C6H12O6 (molar mass: 180.16 g/mol) may refer to:

Inositol oxygenase, also commonly referred to as myo-inositol oxygenase (MIOX), is a non-heme di-iron enzyme that oxidizes myo-inositol to glucuronic acid. The enzyme employs a unique four-electron transfer at its Fe(II)/Fe(III) coordination sites and the reaction proceeds through the direct binding of myo-inositol followed by attack of the iron center by diatomic oxygen. This enzyme is part of the only known pathway for the catabolism of inositol in humans and is expressed primarily in the kidneys. Recent medical research regarding MIOX has focused on understanding its role in metabolic and kidney diseases such as diabetes, obesity and acute kidney injury. Industrially-focused engineering efforts are centered on improving MIOX activity in order to produce glucaric acid in heterologous hosts.

1D-chiro-Inositol is a member of a family of related substances often referred to collectively as "inositol", although that term encompasses several isomers of questionable biological relevance, including 1L-chiro-inositol. myo-Inositol is converted into DCI by an insulin dependent NAD/NADH epimerase enzyme. It is known to be an important secondary messenger in insulin signal transduction. DCI accelerates the dephosphorylation of glycogen synthase and pyruvate dehydrogenase, rate limiting enzymes of non-oxidative and oxidative glucose disposal. DCI may act to bypass defective normal epimerization of myo-inositol to DCI associated with insulin resistance and at least partially restore insulin sensitivity and glucose disposal. One pilot study found males taking it had increased androgens and reduced estrogen.
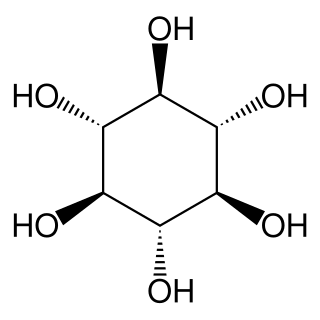
scyllo-Inositol is one of the stereoisomers of inositol. It is also known as scyllitol, cocositol, quercinitol, and 1,3,5/2,4,6-hexahydroxycyclohexane. scyllo-Inositol is a naturally occurring plant sugar alcohol found most abundantly in the coconut palm.
In enzymology, a D-pinitol dehydrogenase (EC 1.1.1.142) is an enzyme that catalyzes the chemical reaction

Inositol 1,4,5-trisphosphate receptor type 1 is a protein that in humans is encoded by the ITPR1 gene.

The enzyme Inositol phosphate-phosphatase is of the phosphodiesterase family of enzymes. It is involved in the phosphophatidylinositol signaling pathway, which affects a wide array of cell functions, including but not limited to, cell growth, apoptosis, secretion, and information processing. Inhibition of inositol monophosphatase may be key in the action of lithium in treating bipolar disorder, specifically manic depression.

Src homology 2 (SH2) domain containing inositol polyphosphate 5-phosphatase 1(SHIP1) is an enzyme with phosphatase activity. SHIP1 is structured by multiple domain and is encoded by the INPP5D gene in humans. SHIP1 is expressed predominantly by hematopoietic cells but also, for example, by osteoblasts and endothelial cells. This phosphatase is important for the regulation of cellular activation. Not only catalytic but also adaptor activities of this protein are involved in this process. Its movement from the cytosol to the cytoplasmic membrane, where predominantly performs its function, is mediated by tyrosine phosphorylation of the intracellular chains of cell surface receptors that SHIP1 binds. Insufficient regulation of SHIP1 leads to different pathologies.
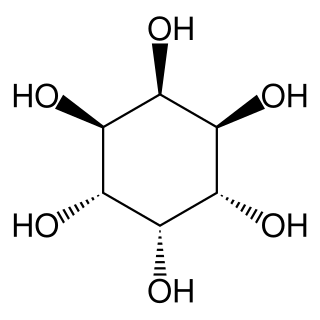
neo-Inositol is one of the stereoisomers of inositol. It is one of the nine isomeric forms of cyclohexanehexol; a group of small and chemically very stable polar molecules that have versatile properties. This stereoisomer is naturally occurring, but only in small amounts. It is also known as (1s,2R,3R,4s,5S,6S)-cyclohexane-1,2,3,4,5,6-hexol or 1,2,3/4,5,6-cyclohexanehexol in the IUPAC naming system.
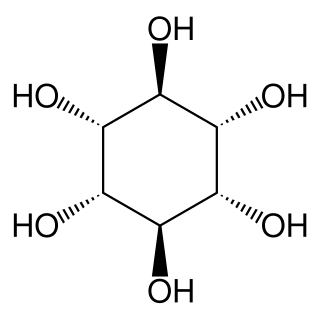
muco-Inositol is a critically important chemical in the gustatory (taste) modality of the mammalian nervous system. The generic form is coupled to a phospholipid of the outer lemma of the sensory neurons associated with the sodium ion sensitive channel of gustation.
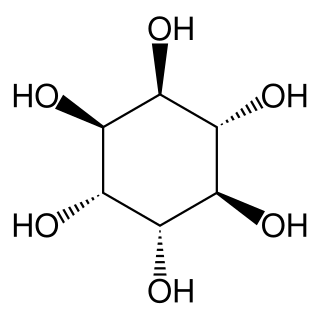
1L-chiro-Inositol (L-chiro-Inositol) is one of the isomers of inositol.
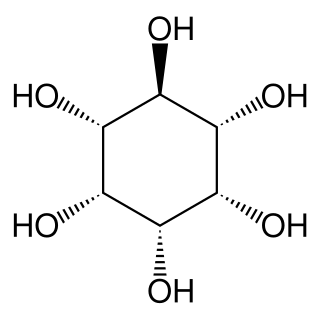
Epi-Inositol is one of the stereoisomers of inositol.

allo-Inositol is a stereoisomer of inositol.

In organic chemistry, a cyclitol is a cycloalkane containing at least three hydroxyl, each attached to a different ring carbon atom. The general formula for an unsubstituted cyclitol is C
nH
2n-x(OH)
x or C
nH
2nO
x where 3 ≤ x ≤ n.

Pinpollitol is a cyclitol. It is a di-O-methyl-(+)-chiro-inositol that can be isolated from Pinus radiata.















