 | |
| Names | |
|---|---|
| IUPAC name neo-Inositol [1] | |
| Systematic IUPAC name (1R,2R,3s,4S,5S,6s)-cyclohexane-1,2,3,4,5,6-hexol | |
| Other names (1s,2R,3R,4s,5S,6S)-cyclohexane-1,2,3,4,5,6-hexol; 1,2,3/4,5,6-cyclohexanehexol [2] | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| UNII | |
| |
| |
| Properties | |
| C6H12O6 | |
| Molar mass | 180.156 g·mol−1 |
| Appearance | white crystalline solid [3] |
| Density | 1.697 g/ml (from X-ray structure) [4] |
| Melting point | 315 °C; 599 °F; 588 K [5] [6] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Irritating to eyes, respiratory system and skin. [7] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
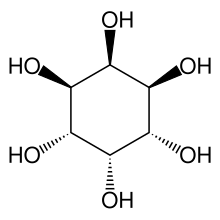
The chemical compound neo-inositol is one of the nine stereoisomers cyclohexane-1,2,3,4,5,6-hexol, the "inositols". Its formula is C6H12O6; the six carbon atoms form a ring, each of them is bonded to a hydrogen atom and a hydroxyl group (–OH). If the ring is assumed horizontal, three consecutive hydroxyls lie above the respective hydrogens, and the other three lie below them.
Contents
- Chemical and physical properties
- Crystal structure
- Synthesis
- Natural occurrence and biological roles
- See also
- References
Like the other stereoisomers, neo-inositol is considered a carbohydrate, specifically a sugar alcohol (to distinguish it from the more familiar ketose and aldose sugars, like glucose). It occurs in nature, but only in small amounts; usually much smaller than those of myo-inositol, the most important stereoisomer. [8]

