 | |
| Names | |
|---|---|
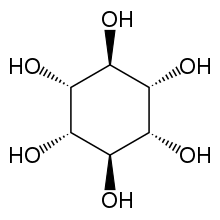
| IUPAC name muco-Inositol [1] | |
| Systematic IUPAC name (1R,2r,3S,4R,5r,6S)-Cyclohexane-1,2,3,4,5,6-hexol | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.983 |
| UNII | |
| |
| |
| Properties | |
| C6H12O6 | |
| Molar mass | 180.156 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Muco-inositol is one of nine stereo-isomers of inositol. It is involved in Na-path sensory transduction,[ citation needed ] and a derivative is viscumitol.
The standardized numbering for atoms in the various inositol isomers has changed significantly since the 1950s. Only literature subsequent to 1988 adopts the modern convention, which is based on phosphorylation patterns in biological systems. [2] In particular, the #1 atom is typically bound with a phosphoester in the hydrated sodium receptor.[ citation needed ]
