Behavioral neuroscience, also known as biological psychology, biopsychology, or psychobiology, is part of the broad, interdisciplinary field of neuroscience, with its primary focus being on the biological and neural mechanisms underlying behavior. Cognitive neuroscience is similar to behavioral neuroscience, in that both fields study the neurobiological functions related to psychology, as in experiences and behaviors. Behavioral neuroscientists examine the biological bases of behavior through research that involves neuroanatomical substrates, environmental and genetic factors, effects of lesions and electrical stimulation, developmental processes, recording electrical activity, neurotransmitters, hormonal influences, chemical components, and the effects of drugs. Important topics of consideration for neuroscientific research in behavior include learning and memory, sensory processes, motivation and emotion, as well as genetic and molecular substrates concerning the biological bases of behavior.

5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.
A receptor activated solely by a synthetic ligand (RASSL) or designer receptor exclusively activated by designer drugs (DREADD), is a class of artificially engineered protein receptors used in the field of chemogenetics which are selectively activated by certain ligands. They are used in biomedical research, in particular in neuroscience to manipulate the activity of neurons.

The endocannabinoid system (ECS) is a biological system composed of endocannabinoids, which are neurotransmitters that bind to cannabinoid receptors, and cannabinoid receptor proteins that are expressed throughout the central nervous system and peripheral nervous system. The endocannabinoid system is still not fully understood, but may be involved in regulating physiological and cognitive processes, including fertility, pregnancy, pre- and postnatal development, various activity of immune system, appetite, pain-sensation, mood, and memory, and in mediating the pharmacological effects of cannabis. The ECS plays an important role in multiple aspects of neural functions, including the control of movement and motor coordination, learning and memory, emotion and motivation, addictive-like behavior and pain modulation, among others.

The p75 neurotrophin receptor (p75NTR) was first identified in 1973 as the low-affinity nerve growth factor receptor (LNGFR) before discovery that p75NTR bound other neurotrophins equally well as nerve growth factor. p75NTR is a neurotrophic factor receptor. Neurotrophic factor receptors bind Neurotrophins including Nerve growth factor, Neurotrophin-3, Brain-derived neurotrophic factor, and Neurotrophin-4. All neurotrophins bind to p75NTR. This also includes the immature pro-neurotrophin forms. Neurotrophic factor receptors, including p75NTR, are responsible for ensuring a proper density to target ratio of developing neurons, refining broader maps in development into precise connections. p75NTR is involved in pathways that promote neuronal survival and neuronal death.

Calcitonin gene-related peptide (CGRP) is a member of the calcitonin family of peptides consisting of calcitonin, amylin, adrenomedullin, adrenomedullin 2 (intermedin) and calcitonin‑receptor‑stimulating peptide. Calcitonin is mainly produced by thyroid C cells whilst CGRP is secreted and stored in the nervous system. This peptide, in humans, exists in two forms: CGRP alpha, and CGRP beta. α-CGRP is a 37-amino acid neuropeptide and is formed by alternative splicing of the calcitonin/CGRP gene located on chromosome 11. β-CGRP is less studied. In humans, β-CGRP differs from α-CGRP by three amino acids and is encoded in a separate, nearby gene. The CGRP family includes calcitonin (CT), adrenomedullin (AM), and amylin (AMY).

Neurotensin is a 13 amino acid neuropeptide that is implicated in the regulation of luteinizing hormone and prolactin release and has significant interaction with the dopaminergic system. Neurotensin was first isolated from extracts of bovine hypothalamus based on its ability to cause a visible vasodilation in the exposed cutaneous regions of anesthetized rats.
In molecular biology, extracellular signal-regulated kinases (ERKs) or classical MAP kinases are widely expressed protein kinase intracellular signalling molecules that are involved in functions including the regulation of meiosis, mitosis, and postmitotic functions in differentiated cells. Many different stimuli, including growth factors, cytokines, virus infection, ligands for heterotrimeric G protein-coupled receptors, transforming agents, and carcinogens, activate the ERK pathway.

Estrogen-related receptor alpha (ERRα), also known as NR3B1, is a nuclear receptor that in humans is encoded by the ESRRA gene. ERRα was originally cloned by DNA sequence homology to the estrogen receptor alpha, but subsequent ligand binding and reporter-gene transfection experiments demonstrated that estrogens did not regulate ERRα. Currently, ERRα is considered an orphan nuclear receptor.

G protein-coupled receptor 55 also known as GPR55 is a G protein-coupled receptor that in humans is encoded by the GPR55 gene.

SCH-58261 is a drug which acts as a potent and selective antagonist for the adenosine receptor A2A, with more than 50x selectivity for A2A over other adenosine receptors. It has been used to investigate the mechanism of action of caffeine, which is a mixed A1 / A2A antagonist, and has shown that the A2A receptor is primarily responsible for the stimulant and ergogenic effects of caffeine, but blockade of both A1 and A2A receptors is required to accurately replicate caffeine's effects in animals. SCH-58261 has also shown antidepressant, nootropic and neuroprotective effects in a variety of animal models, and has been investigated as a possible treatment for Parkinson's disease.
The alpha-7 nicotinic receptor, also known as the α7 receptor, is a type of nicotinic acetylcholine receptor implicated in long-term memory, consisting entirely of α7 subunits. As with other nicotinic acetylcholine receptors, functional α7 receptors are pentameric [i.e., (α7)5 stoichiometry].

Abnormal cannabidiol (Abn-CBD) is a synthetic regioisomer of cannabidiol, which unlike most other cannabinoids produces vasodilator effects, lowers blood pressure, and induces cell migration, cell proliferation and mitogen-activated protein kinase activation in microglia, but without producing any psychoactive or sedative effects. Abn-CBD can be found as an impurity in synthetic cannabidiol.
Gene therapy is being studied for some forms of epilepsy. It relies on viral or non-viral vectors to deliver DNA or RNA to target brain areas where seizures arise, in order to prevent the development of epilepsy or to reduce the frequency and/or severity of seizures. Gene therapy has delivered promising results in early stage clinical trials for other neurological disorders such as Parkinson's disease, raising the hope that it will become a treatment for intractable epilepsy.

25CN-NBOH is a compound indirectly derived from the phenethylamine series of hallucinogens, which was discovered in 2014 at the University of Copenhagen. This compound is notable as one of the most selective agonist ligands for the 5-HT2A receptor yet discovered, with a pKi of 8.88 at the human 5-HT2A receptor and with 100x selectivity for 5-HT2A over 5-HT2C, and 46x selectivity for 5-HT2A over 5-HT2B. A tritiated version of 25CN-NBOH has also been accessed and used for more detailed investigations of the binding to 5-HT2 receptors and autoradiography.
Chemogenetics is the process by which macromolecules can be engineered to interact with previously unrecognized small molecules. Chemogenetics as a term was originally coined to describe the observed effects of mutations on chalcone isomerase activity on substrate specificities in the flowers of Dianthus caryophyllus. This method is very similar to optogenetics; however, it uses chemically engineered molecules and ligands instead of light and light-sensitive channels known as opsins.
The parafacial zone (PZ) is a brain structure located in the brainstem within the medulla oblongata believed to be heavily responsible for non-rapid eye movement (non-REM) sleep regulation, specifically for inducing slow-wave sleep.

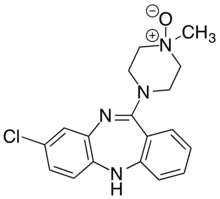
Perlapine, sold under the brand names Hypnodine and Pipnodine, is a hypnotic and sedative of the tricyclic group which is marketed in Japan. It acts primarily as a potent antihistamine, and also has anticholinergic, antiserotonergic, antiadrenergic, and some antidopaminergic activity. The drug has relatively weak affinity for the dopamine D2 receptor (IC50Tooltip Half-maximal inhibitory concentration = 1,803 nM) and, in accordance, is said to be ineffective as an antipsychotic. However, it retains higher affinity for the dopamine D1 receptor (IC50 = 198 nM). Its IC50 values are 19 nM for the α1-adrenergic receptor, 4,945 nM for the α2-adrenergic receptor, and 70 nM for the serotonin 5-HT2A receptor. Perlapine is closely related to clotiapine, clozapine, fluperlapine, loxapine, and tilozepine.
Bryan L. Roth is the Michael Hooker Distinguished Professor of Protein Therapeutics and Translational Proteomics, UNC School of Medicine. He is recognized for his discoveries and inventions in the general areas of molecular pharmacology, GPCR structure, and function and synthetic neurobiology. He is a member of the American Academy of Arts and Sciences (AAAS) and the National Academy of Medicine (NAM)
Fiber photometry is a calcium imaging technique that captures 'bulk' or population-level calcium (Ca2+) activity from specific cell-types within a brain region or functional network in order to study neural circuits Population-level calcium activity can be correlated with behavioral tasks, such as spatial learning, memory recall and goal-directed behaviors. The technique involves the surgical implantation of fiber optics into the brains of living animals. The benefits to researchers are that optical fibers are simpler to implant, less invasive and less expensive than other calcium methods, and there is less weight and stress on the animal, as compared to miniscopes. It also allows for imaging of multiple interacting brain regions and integration with other neuroscience techniques. The limitations of fiber photometry are low cellular and spatial resolution, and the fact that animals must be securely tethered to a rigid fiber bundle, which may impact the naturalistic behavior of smaller mammals such as mice.