Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent activities, corresponding to ~4% of all classified activities. The versatility of PLP arises from its ability to covalently bind the substrate, and then to act as an electrophilic catalyst, thereby stabilizing different types of carbanionic reaction intermediates.

The bacterial outer membrane is found in gram-negative bacteria. Its composition is distinct from that of the inner cytoplasmic cell membrane - among other things, the outer leaflet of the outer membrane of many gram-negative bacteria includes a complex lipopolysaccharide whose lipid portion acts as an endotoxin - and in some bacteria such as E. coli it is linked to the cell's peptidoglycan by Braun's lipoprotein.

Glycosyltransferases are enzymes that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar to a nucleophilic glycosyl acceptor molecule, the nucleophile of which can be oxygen- carbon-, nitrogen-, or sulfur-based.

Perosamine is a mannose-derived 4-aminodeoxysugar produced by some bacteria.

Serine dehydratase or L-serine ammonia lyase (SDH) is in the β-family of pyridoxal phosphate-dependent (PLP) enzymes. SDH is found widely in nature, but its structural and properties vary among species. SDH is found in yeast, bacteria, and the cytoplasm of mammalian hepatocytes. SDH catalyzes is the deamination of L-serine to yield pyruvate, with the release of ammonia.
In enzymology, a GDP-L-fucose synthase (EC 1.1.1.271) is an enzyme that catalyzes the chemical reaction

Threonine ammonia-lyase, also commonly referred to as threonine deaminase or threonine dehydratase, is an enzyme responsible for catalyzing the conversion of L-threonine into alpha-ketobutyrate and ammonia. Alpha-ketobutyrate can be converted into L-isoleucine, so threonine ammonia-lyase functions as a key enzyme in BCAA synthesis. It employs a pyridoxal-5'-phosphate cofactor, similar to many enzymes involved in amino acid metabolism. It is found in bacteria, yeast, and plants, though most research to date has focused on forms of the enzyme in bacteria. This enzyme was one of the first in which negative feedback inhibition by the end product of a metabolic pathway was directly observed and studied. The enzyme serves as an excellent example of the regulatory strategies used in amino acid homeostasis.

In enzymology, a 3-dehydroquinate dehydratase (EC 4.2.1.10) is an enzyme that catalyzes the chemical reaction
In enzymology, a dTDP-glucose 4,6-dehydratase (EC 4.2.1.46) is an enzyme that catalyzes the chemical reaction

In enzymology, a GDP-mannose 4,6-dehydratase (EC 4.2.1.47) is an enzyme that catalyzes the chemical reaction

In enzymology, an imidazoleglycerol-phosphate dehydratase (EC 4.2.1.19) is an enzyme that catalyzes the chemical reaction
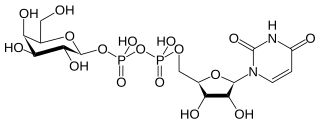
In nucleotide sugar metabolism a group of biochemicals known as nucleotide sugars act as donors for sugar residues in the glycosylation reactions that produce polysaccharides. They are substrates for glycosyltransferases. The nucleotide sugars are also intermediates in nucleotide sugar interconversions that produce some of the activated sugars needed for glycosylation reactions. Since most glycosylation takes place in the endoplasmic reticulum and golgi apparatus, there are a large family of nucleotide sugar transporters that allow nucleotide sugars to move from the cytoplasm, where they are produced, into the organelles where they are consumed.

Guanosine diphosphate mannose or GDP-mannose is a nucleotide sugar that is a substrate for glycosyltransferase reactions in metabolism. This compound is a substrate for enzymes called mannosyltransferases.
In enzymology, a glucomannan 4-beta-mannosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a lipopolysaccharide glucosyltransferase II is an enzyme that catalyzes the chemical reaction

In enzymology, a nicotinate-nucleotide-dimethylbenzimidazole phosphoribosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, the committed step is an effectively irreversible enzymatic reaction that occurs at a branch point during the biosynthesis of some molecules. As the name implies, after this step, the molecules are "committed" to the pathway and will ultimately end up in the pathway's final product. The first committed step should not be confused with the rate-determining step, which is the slowest step in a reaction or pathway. However, it is sometimes the case that the first committed step is in fact the rate-determining step as well.
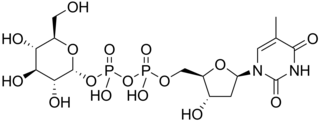
Thymidine diphosphate glucose is a nucleotide-linked sugar consisting of deoxythymidine diphosphate linked to glucose. It is the starting compound for the syntheses of many deoxysugars.
Core oligosaccharide is a short chain of sugar residues within Gram-negative lipopolysaccharide (LPS). Core-OS are highly diverse among bacterial species and even within strains of species
N-glycosyltransferase is an enzyme in prokaryotes which transfers individual hexoses onto asparagine sidechains in substrate proteins, using a nucleotide-bound intermediary, within the cytoplasm. They are distinct from regular N-glycosylating enzymes, which are oligosaccharyltransferases that transfer pre-assembled oligosaccharides. Both enzyme families however target a shared amino acid sequence asparagine—-any amino acid except proline—serine or threonine (N–x–S/T), with some variations.