
A rotaxane is a mechanically interlocked molecular architecture consisting of a dumbbell-shaped molecule which is threaded through a macrocycle. The two components of a rotaxane are kinetically trapped since the ends of the dumbbell are larger than the internal diameter of the ring and prevent dissociation (unthreading) of the components since this would require significant distortion of the covalent bonds.
Viologens are organic compounds with the formula (C5H4NR)2n+. In some viologens, the pyridyl groups are further modified.
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects.
In macromolecular chemistry, a catenane is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. They are conceptually related to other mechanically interlocked molecular architectures, such as rotaxanes, molecular knots or molecular Borromean rings. Recently the terminology "mechanical bond" has been coined that describes the connection between the macrocycles of a catenane. Catenanes have been synthesised in two different ways: statistical synthesis and template-directed synthesis.

In host–guest chemistry, an inclusion compound is a chemical complex in which one chemical compound has a cavity into which a "guest" compound can be accommodated. The interaction between the host and guest involves purely van der Waals bonding. The definition of inclusion compounds is very broad, extending to channels formed between molecules in a crystal lattice in which guest molecules can fit.

In supramolecular chemistry, host–guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host–guest chemistry encompasses the idea of molecular recognition and interactions through non-covalent bonding. Non-covalent bonding is critical in maintaining the 3D structure of large molecules, such as proteins and is involved in many biological processes in which large molecules bind specifically but transiently to one another.

Molecular machines are a class of molecules typically described as an assembly of a discrete number of molecular components intended to produce mechanical movements in response to specific stimuli, mimicking macromolecular devices such as switches and motors. Naturally occurring or biological molecular machines are responsible for vital living processes such as DNA replication and ATP synthesis. Kinesins and ribosomes are examples of molecular machines, and they often take the form of multi-protein complexes. For the last several decades, scientists have attempted, with varying degrees of success, to miniaturize machines found in the macroscopic world. The first example of an artificial molecular machine (AMM) was reported in 1994, featuring a rotaxane with a ring and two different possible binding sites. In 2016 the Nobel Prize in Chemistry was awarded to Jean-Pierre Sauvage, Sir J. Fraser Stoddart, and Bernard L. Feringa for the design and synthesis of molecular machines.

In chemistry, cryptands are a family of synthetic, bicyclic and polycyclic, multidentate ligands for a variety of cations. The Nobel Prize for Chemistry in 1987 was given to Donald J. Cram, Jean-Marie Lehn, and Charles J. Pedersen for their efforts in discovering and determining uses of cryptands and crown ethers, thus launching the now flourishing field of supramolecular chemistry. The term cryptand implies that this ligand binds substrates in a crypt, interring the guest as in a burial. These molecules are three-dimensional analogues of crown ethers but are more selective and strong as complexes for the guest ions. The resulting complexes are lipophilic.
A calixarene is a macrocycle or cyclic oligomer based on a methylene-linked phenols. With hydrophobic cavities that can hold smaller molecules or ions, calixarenes belong to the class of cavitands known in host–guest chemistry.

In chemistry, a cavitand is a container-shaped molecule. The cavity of the cavitand allows it to engage in host–guest chemistry with guest molecules of a complementary shape and size. The original definition proposed by Cram includes many classes of molecules: cyclodextrins, calixarenes, pillararenes and cucurbiturils. However, modern usage in the field of supramolecular chemistry specifically refers to cavitands formed on a resorcinarene scaffold by bridging adjacent phenolic units. The simplest bridging unit is methylene, although dimethylene, trimethylene, benzal, xylyl, pyridyl, 2,3-disubstituted-quinoxaline, o-dinitrobenzyl, dialkylsilylene, and phosphonates are known. Cavitands that have an extended aromatic bridging unit, or an extended cavity containing 3 rows of aromatic rings are referred to as deep-cavity cavitands and have broad applications in host-guest chemistry. These types of cavitands were extensively investigated by Rebek, and Gibb, among others.

In host–guest chemistry, a carcerand is a host molecule that completely entraps its guest so that it will not escape even at high temperatures. This type of molecule was first described in 1985 by Donald J. Cram and coworkers. The complexes formed by a carcerand with permanently imprisoned guests are called carceplexes.
In chemistry, a resorcinarene is a macrocycle, or a cyclic oligomer, based on the condensation of resorcinol (1,3-dihydroxybenzene) and an aldehyde. Resorcinarenes are a type of calixarene. Other types of resorcinarenes include the related pyrogallolarenes and octahydroxypyridines, derived from pyrogallol and 2,6-dihydroxypyridine, respectively.
In chemistry, mechanically interlocked molecular architectures (MIMAs) are molecules that are connected as a consequence of their topology. This connection of molecules is analogous to keys on a keychain loop. The keys are not directly connected to the keychain loop but they cannot be separated without breaking the loop. On the molecular level, the interlocked molecules cannot be separated without the breaking of the covalent bonds that comprise the conjoined molecules; this is referred to as a mechanical bond. Examples of mechanically interlocked molecular architectures include catenanes, rotaxanes, molecular knots, and molecular Borromean rings. Work in this area was recognized with the 2016 Nobel Prize in Chemistry to Bernard L. Feringa, Jean-Pierre Sauvage, and J. Fraser Stoddart.
A molecular switch is a molecule that can be reversibly shifted between two or more stable states. The molecules may be shifted between the states in response to environmental stimuli, such as changes in pH, light, temperature, an electric current, microenvironment, or in the presence of ions and other ligands. In some cases, a combination of stimuli is required. The oldest forms of synthetic molecular switches are pH indicators, which display distinct colors as a function of pH. Currently synthetic molecular switches are of interest in the field of nanotechnology for application in molecular computers or responsive drug delivery systems. Molecular switches are also important in biology because many biological functions are based on it, for instance allosteric regulation and vision. They are also one of the simplest examples of molecular machines.

Pillararenes are macrocycles composed of hydroquinone or dialkoxybenzene units linked in the para position by methylene bridges. They are structurally similar to the cucurbiturils and calixarenes that play an important part in host–guest chemistry. The first pillararene was the five membered dimethoxypillar[5]arene.
Coordination cages are three-dimensional ordered structures in solution that act as hosts in host–guest chemistry. They are self-assembled in solution from organometallic precursors, and often rely solely on noncovalent interactions rather than covalent bonds. Coordinate bonds are useful in such supramolecular self-assembly because of their versatile geometries. However, there is controversy over calling coordinate bonds noncovalent, as they are typically strong bonds and have covalent character. The combination of a coordination cage and a guest is a type of inclusion compound. Coordination complexes can be used as "nano-laboratories" for synthesis, and to isolate interesting intermediates. The inclusion complexes of a guest inside a coordination cage show intriguing chemistry as well; often, the properties of the cage will change depending on the guest. Coordination complexes are molecular moieties, so they are distinct from clathrates and metal-organic frameworks.

Kim Kimoon is a South Korean chemist and professor in the Department of Chemistry at Pohang University of Science and Technology (POSTECH). He is the first and current director of the Center for Self-assembly and Complexity at the Institute for Basic Science. Kim has authored or coauthored 300 papers which have been cited more than 30,000 times and he holds a number of patents. His work has been published in Nature, Nature Chemistry, Angewandte Chemie, and JACS, among others. He has been a Clarivate Analytics Highly Cited Researcher in the field of chemistry in 2014, 2015, 2016.
Topological inhibitors are rigid three-dimensional molecules of inorganic, organic, and hybrid compounds that form multicentered supramolecular interactions in vacant cavities of protein macromolecules and their complexes.

Supramolecular catalysis is not a well-defined field but it generally refers to an application of supramolecular chemistry, especially molecular recognition and guest binding, toward catalysis. This field was originally inspired by enzymatic system which, unlike classical organic chemistry reactions, utilizes non-covalent interactions such as hydrogen bonding, cation-pi interaction, and hydrophobic forces to dramatically accelerate rate of reaction and/or allow highly selective reactions to occur. Because enzymes are structurally complex and difficult to modify, supramolecular catalysts offer a simpler model for studying factors involved in catalytic efficiency of the enzyme. Another goal that motivates this field is the development of efficient and practical catalysts that may or may not have an enzyme equivalent in nature.

Polyrotaxane is a type of mechanically interlocked molecule consisting of strings and rings, in which multiple rings are threaded onto a molecular axle and prevented from dethreading by two bulky end groups. As oligomeric or polymeric species of rotaxanes, polyrotaxanes are also capable of converting energy input to molecular movements because the ring motions can be controlled by external stimulus. Polyrotaxanes have attracted much attention for decades, because they can help build functional molecular machines with complicated molecular structure.
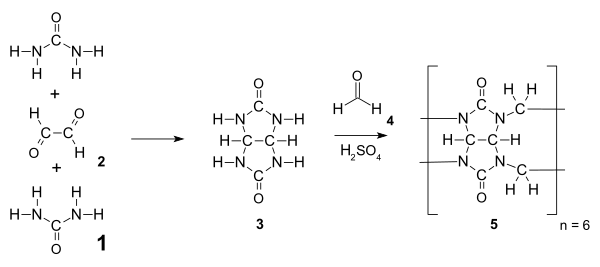
![Computer models of CB[5], CB[6], and CB[7]. Top row is the view into the cavity and the bottom is the side view Models of cucurbiturils.jpg](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d8/Models_of_cucurbiturils.jpg/400px-Models_of_cucurbiturils.jpg)
![Crystal structure of the CB[10]*CB[5] complex including a chloride anion. Cucurbituril gyroscope AngewChemIntEd 2002 v41 p275 hires.png](http://upload.wikimedia.org/wikipedia/commons/thumb/0/0e/Cucurbituril_gyroscope_AngewChemIntEd_2002_v41_p275_hires.png/200px-Cucurbituril_gyroscope_AngewChemIntEd_2002_v41_p275_hires.png)
![Crystal structure of a host-guest complex with a p-xylylenediammonium bound within a cucurbit[6]uril Cucurbit-6-uril ActaCrystallB-Stru 1984 382.jpg](http://upload.wikimedia.org/wikipedia/commons/thumb/9/98/Cucurbit-6-uril_ActaCrystallB-Stru_1984_382.jpg/200px-Cucurbit-6-uril_ActaCrystallB-Stru_1984_382.jpg)









