Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects.

In supramolecular chemistry, host–guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host–guest chemistry encompasses the idea of molecular recognition and interactions through non-covalent bonding. Non-covalent bonding is critical in maintaining the 3D structure of large molecules, such as proteins and is involved in many biological processes in which large molecules bind specifically but transiently to one another.

In host-guest chemistry, cucurbiturils are macrocyclic molecules made of glycoluril monomers linked by methylene bridges. The oxygen atoms are located along the edges of the band and are tilted inwards, forming a partly enclosed cavity (cavitand). The name is derived from the resemblance of this molecule with a pumpkin of the family of Cucurbitaceae.

In chemistry, cryptands are a family of synthetic, bicyclic and polycyclic, multidentate ligands for a variety of cations. The Nobel Prize for Chemistry in 1987 was given to Donald J. Cram, Jean-Marie Lehn, and Charles J. Pedersen for their efforts in discovering and determining uses of cryptands and crown ethers, thus launching the now flourishing field of supramolecular chemistry. The term cryptand implies that this ligand binds substrates in a crypt, interring the guest as in a burial. These molecules are three-dimensional analogues of crown ethers but are more selective and strong as complexes for the guest ions. The resulting complexes are lipophilic.
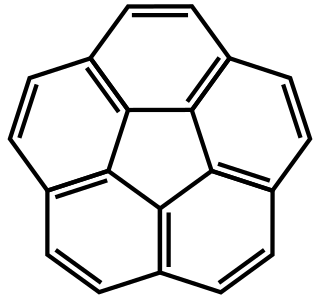
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is [5]circulene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of buckminsterfullerene. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Buckybowls are fragments of buckyballs. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier of 10.2 kcal/mol (42.7 kJ/mol) at −64 °C.
Dynamic covalent chemistry (DCvC) is a synthetic strategy employed by chemists to make complex molecular and supramolecular assemblies from discrete molecular building blocks. DCvC has allowed access to complex assemblies such as covalent organic frameworks, molecular knots, polymers, and novel macrocycles. Not to be confused with dynamic combinatorial chemistry, DCvC concerns only covalent bonding interactions. As such, it only encompasses a subset of supramolecular chemistries.

Achim Müller is a German chemist. He is Professor Emeritus at the Faculty of Chemistry, University of Bielefeld.
In polymer chemistry and materials science, the term "polymer" refers to large molecules whose structure is composed of multiple repeating units. Supramolecular polymers are a new category of polymers that can potentially be used for material applications beyond the limits of conventional polymers. By definition, supramolecular polymers are polymeric arrays of monomeric units that are connected by reversible and highly directional secondary interactions–that is, non-covalent bonds. These non-covalent interactions include van der Waals interactions, hydrogen bonding, Coulomb or ionic interactions, π-π stacking, metal coordination, halogen bonding, chalcogen bonding, and host–guest interaction. The direction and strength of the interactions are precisely tuned so that the array of molecules behaves as a polymer in dilute and concentrated solution, as well as in the bulk.
In chemistry, a halogen bond occurs when there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom in a molecular entity and a nucleophilic region in another, or the same, molecular entity. Like a hydrogen bond, the result is not a formal chemical bond, but rather a strong electrostatic attraction. Mathematically, the interaction can be decomposed in two terms: one describing an electrostatic, orbital-mixing charge-transfer and another describing electron-cloud dispersion. Halogen bonds find application in supramolecular chemistry; drug design and biochemistry; crystal engineering and liquid crystals; and organic catalysis.
Covalent organic frameworks (COFs) are a class of porous polymers that form two- or three-dimensional structures through reactions between organic precursors resulting in strong, covalent bonds to afford porous, stable, and crystalline materials. COFs emerged as a field from the overarching domain of organic materials as researchers optimized both synthetic control and precursor selection. These improvements to coordination chemistry enabled non-porous and amorphous organic materials such as organic polymers to advance into the construction of porous, crystalline materials with rigid structures that granted exceptional material stability in a wide range of solvents and conditions. Through the development of reticular chemistry, precise synthetic control was achieved and resulted in ordered, nano-porous structures with highly preferential structural orientation and properties which could be synergistically enhanced and amplified. With judicious selection of COF secondary building units (SBUs), or precursors, the final structure could be predetermined, and modified with exceptional control enabling fine-tuning of emergent properties. This level of control facilitates the COF material to be designed, synthesized, and utilized in various applications, many times with metrics on scale or surpassing that of the current state-of-the-art approaches.

Young-Tae Chang is a South Korean chemist. He is a professor of chemistry at Pohang University of Science and Technology (POSTECH) and Associate Director under Kim Kimoon at the Center for Self-assembly and Complexity at the Institute for Basic Science located on the POSTECH campus.
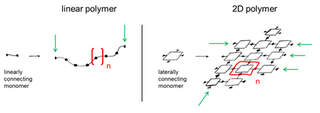
A two-dimensional polymer (2DP) is a sheet-like monomolecular macromolecule consisting of laterally connected repeat units with end groups along all edges. This recent definition of 2DP is based on Hermann Staudinger's polymer concept from the 1920s. According to this, covalent long chain molecules ("Makromoleküle") do exist and are composed of a sequence of linearly connected repeat units and end groups at both termini.

Harry Laurence Anderson is a British chemist in the Department of Chemistry, University of Oxford. He is well known for his contributions in the syntheses of supramolecular systems, exploration of the extraordinary physical properties of large pi-conjugated systems, and synthesis of cyclo[18]carbon. He is a Professor of Chemistry at Keble College, Oxford.

Kwang Soo Kim is a South Korean professor in chemistry, an adjunct professor in physics, and the director of Center for Superfunctional Materials (CSM), of Ulsan National Institute of Science and Technology (UNIST) in South Korea. He received his B.S. and M.S. degrees in Applied Chemistry from Seoul National University and also an M.S. degree in Physics from Korea Advanced Institute of Science and Technology (KAIST) (1975). He obtained his Ph.D. degree from University of California, Berkeley (1982). His research fields include Theoretical/Computational Chemistry/Physics and Experimental Nanosciences.

Philip Alan Gale is an Australian/British chemist, Deputy Dean of Science and Professor of Chemistry at the Faculty of Science, University of Technology Sydney. He is notable for his work on the supramolecular chemistry of anions.

Rajendra Rathore was an organic chemist and professor at Marquette University in Milwaukee, Wisconsin as Pfletschinger-Habermann professor of organic chemistry. He made important contributions in the area of supramolecular chemistry, synthesis of novel electro-active molecules, and drug discovery. Rathore died on 16 February 2018, after complications from chronic pulmonary sarcoidosis.

Dmitrii "Dima" F. Perepichka is the Chair of Chemistry Department and Sir William C. MacDonald Chair Professor in Chemistry at McGill University. His research interest are primarily in the area of organic electronics. He has contributed in the understanding of structural electronics effects of organic conjugated materials at molecular, supramolecular, and macromolecular levels via the study of small molecules, supramolecular (co-)assemblies, polymers, covalent organic frameworks, and on-surface assemblies/polymers.

Helma B. Wennemers is a German organic chemist. She is a professor of organic chemistry at the Swiss Federal Institute of Technology in Zurich.

Jayaraman Sivaguru (Siva) is the Antonia and Marshall Wilson Professor of Chemistry and the Associate Director, Center for Photochemical Sciences at the Department of Chemistry, Bowling Green State University, Bowling Green, Ohio. He is a recipient of 2008 National Science Foundation CAREER Award, 2010 Grammaticakis-Neumann Prize from the Swiss Chemical Society, 2011 young-investigator award from the Inter-American Photochemical Society (I-APS), and 2012-young investigator award from Sigma Xi. His honors also include Excellence in Research award, 2011 Excellence in Teaching award, and the 2012 PeltierAward for Innovation in Teaching. Prof. Siaguru was a visiting young professor at the Global Centre for Excellence at Osaka University, Japan and was a visiting fellow for the Chinese Academy of Sciences President's International Fellowship Initiative in 2018. He is an editor for the Journal of Photochemistry and Photobiology A: Chemistry and from 2020 serves as the co-Editor-in-Chief of Journal of Photochemistry and Photobiology published by Elsevier. He is an international board member of the International Union of Pure and Applied Chemistry (IUPAC) photochemistry symposium.

Transition metal porphyrin complexes are a family of coordination complexes of the conjugate base of porphyrins. Iron porphyrin complexes occur widely in Nature, which has stimulated extensive studies on related synthetic complexes. The metal-porphyrin interaction is a strong one such that metalloporphyrins are thermally robust. They are catalysts and exhibit rich optical properties, although these complexes remain mainly of academic interest.















