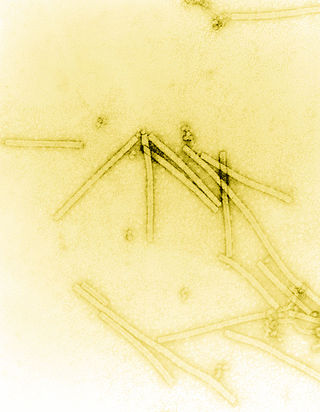
Tobacco mosaic virus (TMV) is a positive-sense single-stranded RNA virus species in the genus Tobamovirus that infects a wide range of plants, especially tobacco and other members of the family Solanaceae. The infection causes characteristic patterns, such as "mosaic"-like mottling and discoloration on the leaves. TMV was the first virus to be discovered. Although it was known from the late 19th century that a non-bacterial infectious disease was damaging tobacco crops, it was not until 1930 that the infectious agent was determined to be a virus. It is the first pathogen identified as a virus. The virus was crystallised by Wendell Meredith Stanley. It has a similar size to the largest synthetic molecule, known as PG5.
Cauliflower mosaic virus (CaMV) is a member of the genus Caulimovirus, one of the six genera in the family Caulimoviridae, which are pararetroviruses that infect plants. Pararetroviruses replicate through reverse transcription just like retroviruses, but the viral particles contain DNA instead of RNA.

Plant viruses are viruses that affect plants. Like all other viruses, plant viruses are obligate intracellular parasites that do not have the molecular machinery to replicate without a host. Plant viruses can be pathogenic to vascular plants.

Tobamovirus is a genus of positive-strand RNA viruses in the family Virgaviridae. Many plants, including tobacco, potato, tomato, and squash, serve as natural hosts. Diseases associated with this genus include: necrotic lesions on leaves. The name Tobamovirus comes from the host and symptoms of the first virus discovered.

Alfalfa mosaic virus (AMV), also known as Lucerne mosaic virus or Potato calico virus, is a worldwide distributed phytopathogen that can lead to necrosis and yellow mosaics on a large variety of plant species, including commercially important crops. It is the only Alfamovirus of the family Bromoviridae. In 1931 Weimer J.L. was the first to report AMV in alfalfa. Transmission of the virus occurs mainly by some aphids, by seeds or by pollen to the seed.
Asparagus virus 1 (AV-1) is one of the nine known viruses that infects asparagus plants. It is a member of the genus Potyvirus in the family Potyviridae. Initially reported by G. L Hein in 1960, it causes no distinct symptoms in asparagus plants. The only known natural plant host is the asparagus. It is spread by aphid vectors, which means that aphids do not cause the AV-1, but they do spread it.

Cucumber mosaic virus (CMV) is a plant pathogenic virus in the family Bromoviridae. This virus has a worldwide distribution and a very wide host range, having the reputation of the widest host range of any known plant virus. It can be transmitted from plant to plant both mechanically by sap and by aphids in a stylet-borne fashion. It can also be transmitted in seeds and by the parasitic weeds, Cuscuta sp. (dodder).

Odontoglossum ringspot virus (ORSV) is a plant pathogenic virus that belongs to the family Virgaviridae. It is one of the most common viruses affecting cultivated orchids, perhaps second only to the Cymbidium mosaic virus. It causes spots on leaves and colored streaks on flowers. If a plant is also infected with the Cymbidium mosaic virus, it can lead to a condition called blossom brown necrotic streak.
Papaya mosaic virus (PapMV) is a plant pathogenic virus in the genus Potexvirus and the family Alphaflexiviridae. PapMV is a filamentous, flexuous rod, 530 nm in length.
Prune dwarf virus (PDV) is an economically important plant pathogenic virus affecting Prunus species globally. PDV is found worldwide due to easy transmission through seed, pollen, and vegetative propagation. The virus is in the family Bromoviridae an important family of plant RNA viruses containing six genera, including Alfamovirus, Ilarvirus, Bromovirus, Amularvirus, Oleavirus, and Cucumovirus. PDV belongs to the genera Ilarvirus. It can cause dwarfism of leaves on certain prune and plum plants. It will also cause yellows in sour cherry, especially when present with Prunus necrotic ringspot virus. There are no known transmission vectors, though the pollen of infected cherry trees has been found to infect other cherry trees a small percent of the time.

Prunus necrotic ringspot virus (PNRSV) is a plant pathogenic virus causing ring spot diseases affecting species of the genus Prunus, as well as other species such as rose and hops. PNRSV is found worldwide due to easy transmission through plant propagation methods and infected seed. The virus is in the family Bromoviridae and genus Ilarvirus. Synonyms of PNRSV include European plum line pattern virus, hop B virus, hop C virus, plum line pattern virus, sour cherry necrotic ringspot virus, and peach ringspot virus.

Potexvirus is a genus of pathogenic viruses in the order Tymovirales, in the family Alphaflexiviridae. Plants serve as natural hosts. There are 48 species in this genus, three of which are assigned to a subgenus. Diseases associated with this genus include: mosaic and ringspot symptoms. The genus name comes from POTato virus X).

Carlavirus, formerly known as the "Carnation latent virus group", is a genus of viruses in the order Tymovirales, in the family Betaflexiviridae. Plants serve as natural hosts. There are 53 species in this genus. Diseases associated with this genus include: mosaic and ringspot symptoms.
Alternanthera mosaic virus (AltMV) is a plant pathogenic virus. AltMV belongs to the virus genus Potexvirus and the virus family Alphaflexiviridae.
Orchid fleck dichorhavirus, commonly called Orchid fleck virus (OFV), is a non-enveloped, segmented, single-stranded (ss) RNA negative-strand virus, transmitted by the false spider mite, Brevipalpus californicus. OFV causes necrotic and chlorotic lesions on the leaves of many genera in the family Orchidaceae.

Fig mosaic emaravirus (FMV) is a segmented, negative sense, single-stranded RNA virus that is determined to be the causal agent of fig mosaic disease (FMD) in fig plants, Ficus carica. It is a member of the genus Emaravirus and order Bunyavirales and is transmitted mainly by the eriophyid mite Aceria ficus. FMV can cause a range of symptoms varying in severity, including leaf chlorosis, deformity, and mosaic or discoloration patterns, as well as premature fruit drop.

Emaravirus is a genus of negative-strand RNA viruses which infect plants. The plant virus group is the sole genus in the family Fimoviridae. The genus has 21 species.
Cocoa necrosis virus (CoNV) is a plant pathogenic virus of the genus nepovirus that infects Theobroma cacao en natura causing cacao necrosis disease. CoNV is considered synonymous with Strain S of cacao swollen shoot virus. Unlike Cacao swollen shoot virus, it is not transmitted by mealybugs nor vectored by aphids, beetles, or leafhoppers that also commonly infest cacao. It is serologically, distantly related to Tomato black ring virus and very distantly related to Grapevine chrome mosaic virus.

Carnation Italian Ringspot Virus (CIRV) is a plant virus that impacts carnation plants. These flowers are a popular choice in ornamental flower arrangements. This article will provide an overview of CIRV. This will include the history of the virus, information on transmission, symptoms, and characteristics, and research about how it relates to plant physiology.
The cardamom mosaic virus (CdMV) is a mosaic virus that affects the production of green cardamom (E. cardamomum). It is a member of the genus Macluravirus (recognized under the family Potyviridae by ICTV in 1988), and is transmitted through aphids (P.caladii) and infected rhizomes, the former in a non-persistent manner.













