Halocarbon compounds are chemical compounds in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms resulting in the formation of organofluorine compounds, organochlorine compounds, organobromine compounds, and organoiodine compounds. Chlorine halocarbons are the most common and are called organochlorides.

Irwin Allan Rose was an American biologist. Along with Aaron Ciechanover and Avram Hershko, he was awarded the 2004 Nobel Prize in Chemistry for the discovery of ubiquitin-mediated protein degradation.
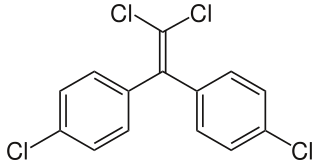
Dichlorodiphenyldichloroethylene (DDE) is a chemical compound formed by the loss of hydrogen chloride (dehydrohalogenation) from DDT, of which it is one of the more common breakdown products. Due to DDT's massive prevalence in society and agriculture during the mid 20th century, DDT and DDE are still widely seen in animal tissue samples. DDE is particularly dangerous because it is fat-soluble like other organochlorines; thus, it is rarely excreted from the body, and concentrations tend to increase throughout life. The major exception is the excretion of DDE in breast milk, which transfers a substantial portion of the mother's DDE burden to the young animal or child. Along with accumulation over an organism's lifetime, this stability leads to bioaccumulation in the environment, which amplifies DDE's negative effects.
This is the list of extremely hazardous substances defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act. The list can be found as an appendix to 40 CFR 355. Updates as of 2006 can be seen on the Federal Register, 71 FR 47121.

HPTE, also known as hydroxychlor, p,p'-hydroxy-DDT, or 2,2-bis(4-hydroxyphenyl)-1,1,1-trichloroethane, is a metabolite of methoxychlor, a synthetic insecticide related to DDT. Like bisphenol A with similar chemical structure, HPTE is an endocrine disruptor which has estrogenic activity, and also inhibits Cholesterol side-chain cleavage enzyme and 3α-hydroxysteroid dehydrogenase (3α-HSD).
In enzymology, a lignin peroxidase (EC 1.11.1.14) is an enzyme that catalyzes the chemical reaction
In enzymology, an inositol-3-phosphate synthase is an enzyme that catalyzes the chemical reaction
The enzyme ethanolamine ammonia-lyase (EC 4.3.1.7) catalyzes the chemical reaction
The enzyme glycosylphosphatidylinositol diacylglycerol-lyase catalyzes the reaction

In enzymology, an aspartate 4-decarboxylase (EC 4.1.1.12) is an enzyme that catalyzes the chemical reaction
The enzyme tagatose-bisphosphate aldolase catalyzes the chemical reaction

The enzyme threonine aldolase is an enzyme that catalyzes the chemical reaction
The enzyme tryptophanase (EC 4.1.99.1) catalyzes the chemical reaction

In enzymology, a formate—tetrahydrofolate ligase is an enzyme that catalyzes the chemical reaction

The enzyme imidazoleglycerol-phosphate dehydratase (EC 4.2.1.19) catalyzes the chemical reaction
Pectate lyase is an enzyme involved in the maceration and soft rotting of plant tissue. Pectate lyase is responsible for the eliminative cleavage of pectate, yielding oligosaccharides with 4-deoxy-α-D-mann-4-enuronosyl groups at their non-reducing ends. The protein is maximally expressed late in pollen development. It has been suggested that the pollen expression of pectate lyase genes might relate to a requirement for pectin degradation during pollen tube growth.
The enzyme xylonate dehydratase (EC 4.2.1.82) catalyzes the chemical reaction:
In enzymology, a beta-galactoside alpha-2,6-sialyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a myosin-heavy-chain kinase is an enzyme that catalyzes the chemical reaction
Thymidylate kinase catalyzes the phosphorylation of thymidine 5'-monophosphate (dTMP) to form thymidine 5'-diphosphate (dTDP) in the presence of ATP and magnesium:











