
Datura is a genus of nine species of poisonous, vespertine-flowering plants belonging to the family Solanaceae. They are commonly known as thornapples or jimsonweeds, but are also known as devil's trumpets. Other English common names include moonflower, devil's weed, and hell's bells. All species of Datura are poisonous and potentially psychoactive, especially their seeds and flowers, which can cause respiratory depression, arrhythmias, fever, delirium, hallucinations, anticholinergic syndrome, psychosis, and even death if taken internally. Due to their effects and symptoms, they have occasionally been used not only as a poison, but also as hallucinogens by various groups throughout history. Traditionally, psychoactive administration of Datura species has often been associated with witchcraft and sorcery or similar practices in many cultures, including the Western world. Certain common Datura species have also been used ritualistically as entheogens by some Native American groups. Nonpsychoactive use of the plant is usually done for medicinal purposes, and the alkaloids present in plants of the Datura genus have long been considered traditional medicines in both the New and Old Worlds due to the presence of the alkaloids scopolamine and atropine, which are also produced by Old World plants such as Hyoscyamus niger, Atropa belladonna, and Mandragora officinarum.

Datura stramonium, known by the common names thorn apple, jimsonweed or devil's snare, is a species of flowering plant in the nightshade family Solanaceae. Its likely origin was in Central America, and it has been introduced in many world regions. It is an aggressive invasive weed in temperate climates across the world. D. stramonium has frequently been employed in traditional medicine to treat a variety of ailments. It has also been used as a hallucinogen, taken entheogenically to cause intense visions. It is unlikely ever to become a major drug of abuse owing to effects upon both mind and body frequently perceived subjectively as highly unpleasant, giving rise to a state of profound and long-lasting disorientation with a potentially fatal outcome. It contains tropane alkaloids which are responsible for the deliriant effects, and may be severely toxic.

Vernicia fordii, usually known as the tung tree is a species of Vernicia in the spurge family native to southern China, Burma, and northern Vietnam. It is a small to medium-sized deciduous tree growing to 20 m tall, with a spreading crown. The bark is smooth and thin, and bleeds latex if cut. The leaves are alternate, simple, 4.5–25 cm long and 3.5–22 cm broad, heart-shaped or with three shallow, maple-like lobes, green above and below, red conspicuous glands at the base of the leaf, and with a 5.5–26 cm long petiole. The flowers are 2.5–3.5 cm diameter, with five pale pink to purple petals with streaks of darker red or purple in the throat; it is monoecious with individual flowers either male or female, but produced together in the inflorescences. The flowers appear before or with the leaves in loose, terminal clusters. The fruit is a hard, woody pear-shaped berry 4–6 cm long and 3–5 cm diameter, containing four or five large, oily seeds; it is green initially, becoming dull brown when ripe in autumn.

Cuscohygrine is a pyrrolidine alkaloid found in coca. It can also be extracted from plants of the family Solanaceae, including Atropa belladonna, Datura innoxia and Datura stramonium. Cuscohygrine usually occurs along with other, more potent alkaloids such as atropine or cocaine.

Datura innoxia, known as pricklyburr, recurved thorn-apple, downy thorn-apple, Indian-apple, lovache, moonflower, nacazcul, toloatzin, toloaxihuitl, tolguache or toloache, is a species of flowering plant in the family Solanaceae. It is more rarely called sacred datura, a common name which is applied more often to the closely related Datura wrightii. It is native to the Southwestern United States, Central and South America, and introduced in Africa, Asia, Australia and Europe. The scientific name is often cited as D. innoxia. When English botanist Philip Miller first described the species in 1768, he misspelled the Latin word innoxia (inoffensive) when naming it D. inoxia. The name Datura meteloides was for some time erroneously applied to some members of the species, but that name has now been abandoned.

Triterpenes are a class of chemical compounds composed of three terpene units with the molecular formula C30H48; they may also be thought of as consisting of six isoprene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids.

Terminalia chebula, commonly known as black- or chebulic myrobalan, is a species of Terminalia, native to South Asia from India and Nepal east to southwest China (Yunnan), and south to Sri Lanka, Malaysia, and Vietnam.
Gymnemic acids are a class of chemical compounds isolated from the leaves of Gymnema sylvestre (Asclepiadaceae). They are anti-sweet compounds, or sweetness inhibitors. After chewing the leaves, solutions sweetened with sugar taste like water.
In enzymology, a tropinone reductase I (EC 1.1.1.206) is an enzyme that catalyzes the chemical reaction
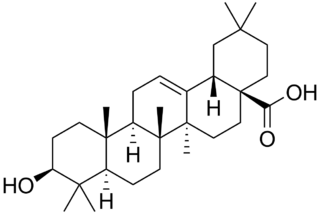
Oleanolic acid or oleanic acid is a naturally occurring pentacyclic triterpenoid related to betulinic acid. It is widely distributed in food and plants where it exists as a free acid or as an aglycone of triterpenoid saponins.
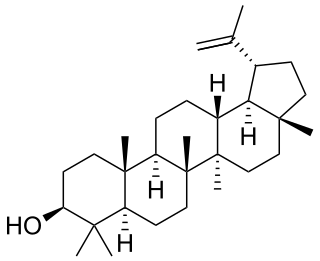
Lupeol is a pharmacologically active pentacyclic triterpenoid. It has several potential medicinal properties, like anticancer and anti-inflammatory activity.

The amyrins are three closely related natural chemical compounds of the triterpene class. They are designated α-amyrin (ursane skeleton), β-amyrin (oleanane skeleton) and δ-amyrin. Each is a pentacyclic triterpenol with the chemical formula C30H50O. They are widely distributed in nature and have been isolated from a variety of plant sources such as epicuticular wax. In plant biosynthesis, α-amyrin is the precursor of ursolic acid and β-amyrin is the precursor of oleanolic acid. All three amyrins occur in the surface wax of tomato fruit. α-Amyrin is found in dandelion coffee.
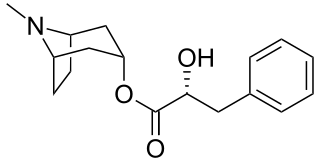
Littorine is a tropane alkaloid found in a variety of plants including Datura and Atropa belladonna. It is closely related in chemical structure to atropine, hyoscyamine, and scopolamine, which all share a common biosynthetic pathway.

Celastrol (tripterine) is a chemical compound isolated from the root extracts of Tripterygium wilfordii and Tripterygium regelii. Celastrol is a pentacyclic nortriterpen quinone and belongs to the family of quinone methides. In mice, celastrol is an NR4A1 agonist that alleviates inflammation and induces autophagy. Also in mice, celastrol increase expression of IL1R1, which is the receptor for the cytokine interleukin-1 (IL-1). IL1R1 knock-out mice exposed to celastrol exhibit no leptin-sensitizing or anti-obesity effect.

Fraxetin is an O-methylated coumarin. It can be found in Fraxinus rhynchophylla and seeds of Datura stramonium. Fraxin is a glucoside of fraxetin.
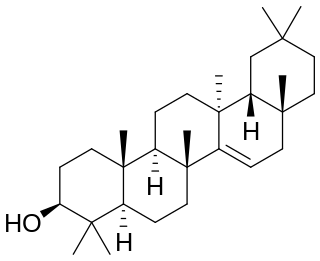
Taraxerol is a naturally-occurring pentacyclic triterpenoid. It exists in various higher plants, including Taraxacum officinale (Asteraceae), Alnus glutinosa (Betulaceae), Litsea dealbata (Lauraceae), Skimmia spp. (Rutaceae), Dorstenia spp. (Moraceae), Maytenus spp. (Celastraceae), and Alchornea latifolia (Euphobiaceae). Taraxerol was named "alnulin" when it was first isolated in 1923 from the bark of the grey alder by Zellner and Röglsperger. It also had the name "skimmiol" when Takeda and Yosiki isolated it from Skimmia (Rutaceae). A large number of medicinal plants are known to have this compound in their leaves, roots or seed oil.
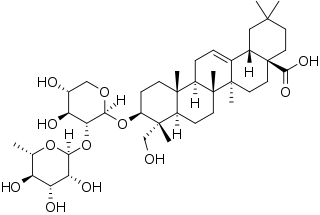
α-Hederin (alpha-hederin) is a water-soluble pentacyclic triterpenoid saponin found in the seeds of Nigella sativa and leaves of Hedera helix.

Tetrahymanol is a gammacerane-type membrane lipid first found in the marine ciliate Tetrahymena pyriformis. It was later found in other ciliates, fungi, ferns, and bacteria. After being deposited in sediments that compress into sedimentary rocks over millions of years, tetrahymanol is dehydroxylated into gammacerane. Gammacerane has been interpreted as a proxy for ancient water column stratification.

Daturaolone is a triterpene found in Datura species such as Datura stramonium and Datura innoxia.

Methylecgonine is a prominent tropane alkaloid found in coca leaves. It is metabolite of cocaine, and maybe is used as precursor for it. It also occurs as minor alkaloid in roots of many Datura species such as Datura stramonium and Datura innoxia.


















