
Beta-lactamases (β-lactamases) are enzymes produced by bacteria that provide multi-resistance to beta-lactam antibiotics such as penicillins, cephalosporins, cephamycins, monobactams and carbapenems (ertapenem), although carbapenems are relatively resistant to beta-lactamase. Beta-lactamase provides antibiotic resistance by breaking the antibiotics' structure. These antibiotics all have a common element in their molecular structure: a four-atom ring known as a beta-lactam (β-lactam) ring. Through hydrolysis, the enzyme lactamase breaks the β-lactam ring open, deactivating the molecule's antibacterial properties.

Datura is a genus of nine species of highly poisonous, vespertine-flowering plants belonging to the nightshade family (Solanaceae). They are commonly known as thornapples or jimsonweeds, but are also known as devil's trumpets. Other English common names include moonflower, devil's weed, and hell's bells. All species of Datura are extremely poisonous and psychoactive, especially their seeds and flowers, which can cause respiratory depression, arrhythmias, fever, delirium, hallucinations, anticholinergic syndrome, psychosis, and death if taken internally.

Datura stramonium, known by the common names thorn apple, jimsonweed, devil's snare, or devil's trumpet, is a poisonous flowering plant of the nightshade family Solanaceae. It is a species belonging to the Datura genus and Daturae tribe. Its likely origin was in Central America, and it has been introduced in many world regions. It is an aggressive invasive weed in temperate climates and tropical climates across the world. D. stramonium has frequently been employed in traditional medicine to treat a variety of ailments. It has also been used as a hallucinogen, taken entheogenically to cause intense, sacred or occult visions. It is unlikely ever to become a major drug of abuse owing to effects upon both mind and body frequently perceived as being highly unpleasant, giving rise to a state of profound and long-lasting disorientation or delirium with a potentially fatal outcome. It contains tropane alkaloids which are responsible for the psychoactive effects, and may be severely toxic.

Deliriants are a subclass of hallucinogen. The term was coined in the early 1980s to distinguish these drugs from psychedelics such as LSD and dissociatives such as ketamine, due to their primary effect of causing delirium, as opposed to the more lucid and less disturbed states produced by other types of hallucinogens. The term generally refers to anticholinergic drugs, which are substances that inhibit the function of the neurotransmitter acetylcholine. Common examples of deliriants include plants of the genera Datura and Brugmansia as well as higher than recommended dosages of diphenhydramine (Benadryl). A number of plant deliriants such as that of the Solanaceae family, particularly in the Americas have been used by some indigenous cultures to reach delirious and altered states for traditions or rituals, such as rites of passage, divination or communicating with the ancestors. Despite their long history of use, deliriants are the least-studied class of hallucinogens in terms of their behavioral and neurological effects.

Enoxolone is a pentacyclic triterpenoid derivative of the beta-amyrin type obtained from the hydrolysis of glycyrrhizic acid, which was obtained from the herb liquorice.

Solanum nigrum, the European black nightshade or simply black nightshade or blackberry nightshade, is a species of flowering plant in the family Solanaceae, native to Eurasia and introduced in the Americas, Australasia, and South Africa. Ripe berries and cooked leaves of edible strains are used as food in some locales, and plant parts are used as a traditional medicine. Some other species may also be referred to as "black nightshade".

Verbena officinalis, the common vervain or common verbena, is a perennial herb native to Europe. It grows up to 70 cm high, with an upright habitus. The lobed leaves are toothed, and the delicate spikes hold clusters of two-lipped mauve flowers.

A phytase is any type of phosphatase enzyme that catalyzes the hydrolysis of phytic acid – an indigestible, organic form of phosphorus that is found in many plant tissues, especially in grains and oil seeds – and releases a usable form of inorganic phosphorus. While phytases have been found to occur in animals, plants, fungi and bacteria, phytases have been most commonly detected and characterized from fungi.
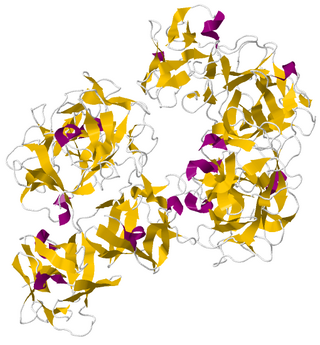
Fascin is an actin bundling protein.
Alternaria carthami is a necrotrophic plant pathogen of safflower. The fungus is in the order Pleosporales and family Pleosporaceae. It was first isolated in India, has spread globally and can have devastating effects on safflower yield, and resultant oilseed production. A. carthami is known to be seed-borne and appears as irregular brown lesions on safflower leaves and stems.
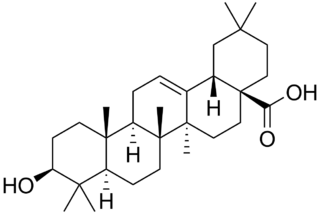
Oleanolic acid or oleanic acid is a naturally occurring pentacyclic triterpenoid related to betulinic acid. It is widely distributed in food and plants where it exists as a free acid or as an aglycone of triterpenoid saponins.

Withanolides are a group of at least 300 naturally occurring steroids built on an ergostane skeleton. They occur as secondary metabolites primarily in genera of the Nightshade family, for example in the tomatillo.
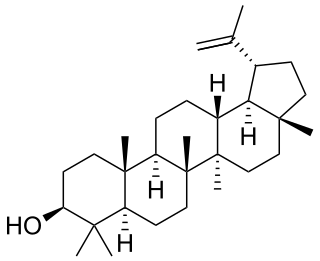
Lupeol is a pharmacologically active pentacyclic triterpenoid. It has several potential medicinal properties, like anticancer and anti-inflammatory activity.

The amyrins are three closely related natural chemical compounds of the triterpene class. They are designated α-amyrin (ursane skeleton), β-amyrin (oleanane skeleton) and δ-amyrin. Each is a pentacyclic triterpenol with the chemical formula C30H50O. They are widely distributed in nature and have been isolated from a variety of plant sources such as epicuticular wax. In plant biosynthesis, α-amyrin is the precursor of ursolic acid and β-amyrin is the precursor of oleanolic acid. All three amyrins occur in the surface wax of tomato fruit. α-Amyrin is found in dandelion coffee.
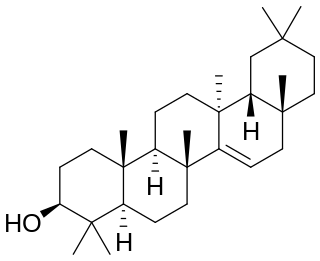
Taraxerol is a naturally-occurring pentacyclic triterpenoid. It exists in various higher plants, including Taraxacum officinale (Asteraceae), Alnus glutinosa (Betulaceae), Litsea dealbata (Lauraceae), Skimmia spp. (Rutaceae), Dorstenia spp. (Moraceae), Maytenus spp. (Celastraceae), and Alchornea latifolia (Euphobiaceae). Taraxerol was named "alnulin" when it was first isolated in 1923 from the bark of the grey alder by Zellner and Röglsperger. It also had the name "skimmiol" when Takeda and Yosiki isolated it from Skimmia (Rutaceae). A large number of medicinal plants are known to have this compound in their leaves, roots or seed oil.

The Solanaceae, or the nightshades, are a family of flowering plants that ranges from annual and perennial herbs to vines, lianas, epiphytes, shrubs, and trees, and includes a number of agricultural crops, medicinal plants, spices, weeds, and ornamentals. Many members of the family contain potent alkaloids, and some are highly toxic, but many—including tomatoes, potatoes, eggplant, bell and chili peppers—are used as food. The family belongs to the order Solanales, in the asterid group and class Magnoliopsida (dicotyledons). The Solanaceae consists of about 98 genera and some 2,700 species, with a great diversity of habitats, morphology and ecology.

Botryosphaeran is an exopolysaccharide (EPS) produced by the ascomyceteous fungus Botryosphaeria rhodina. Characterization of the chemical structure of botryosphaeran showed this EPS to be a (1→3)(1→6)-β-D-glucan. This particular β-glucan can be produced by several strains of Botryosphaeria rhodina that include: MAMB-05, DABAC-P82, and RCYU 30101. Botryosphaeran exhibits interesting rheological properties and novel biological functions including hypoglycaemia, hypocholesterolaemia, anti-atheroslerosis and anti-cancer activity, with potential commercial applications. Three cosmetic products formulated with botryosphaeran have been developed to promote skin health and treat skin conditions for future intended commercialization purposes.
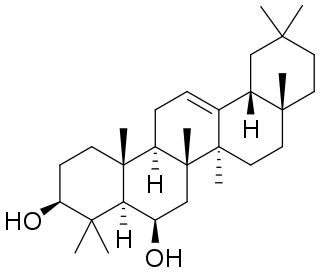
Daturadiol is a pentacyclic triterpenoid found in Datura species including Datura stramonium and Datura innoxia. It is also found in non-Solanaceae plants such as Vernicia fordii and Terminalia brasiliensis.

Methylecgonine, also known as ecgonine methylester is a prominent tropane alkaloid found in coca leaves. It is metabolite of cocaine, and may be used as a precursor for it. It also occurs as minor alkaloid in roots of many Datura species such as Datura stramonium and Datura innoxia.
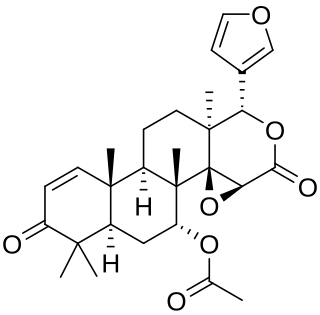
Gedunin is a pentacyclic triterpenoid with the molecular formula C28H34 O7. It is most notably found in Azadirachta indica, but is a constituent of several other plants. Gedunin shows therapeutic potential in the treatment of leukemia, and Parkinson's disease.



















