Cyclopentadiene is an organic compound with the formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−.
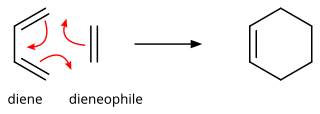
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted mechanism. More specifically, it is classified as a thermally-allowed [4+2] cycloaddition with Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials. The underlying concept has also been applied to π-systems involving heteroatoms, such as carbonyls and imines, which furnish the corresponding heterocycles; this variant is known as the hetero-Diels–Alder reaction. The reaction has also been generalized to other ring sizes, although none of these generalizations have matched the formation of six-membered rings in terms of scope or versatility. Because of the negative values of ΔH° and ΔS° for a typical Diels–Alder reaction, the microscopic reverse of a Diels–Alder reaction becomes favorable at high temperatures, although this is of synthetic importance for only a limited range of Diels-Alder adducts, generally with some special structural features; this reverse reaction is known as the retro-Diels–Alder reaction.
In chemical synthesis, click chemistry is a class of biocompatible small molecule reactions commonly used in bioconjugation, allowing the joining of substrates of choice with specific biomolecules. Click chemistry is not a single specific reaction, but describes a way of generating products that follow examples in nature, which also generates substances by joining small modular units. In many applications, click reactions join a biomolecule and a reporter molecule. Click chemistry is not limited to biological conditions: the concept of a "click" reaction has been used in chemoproteomic, pharmacological, and various biomimetic applications. However, they have been made notably useful in the detection, localization and qualification of biomolecules.
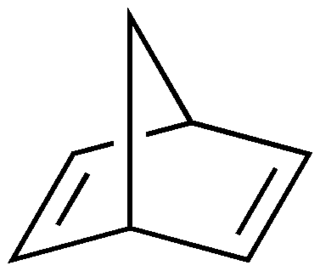
Norbornadiene is an organic compound and a bicyclic hydrocarbon. Norbornadiene is of interest as a metal-binding ligand, whose complexes are useful for homogeneous catalysis. It has been intensively studied owing to its high reactivity and distinctive structural property of being a diene that cannot isomerize. Norbornadiene is also a useful dienophile in Diels-Alder reactions.
[n]Radialenes are alicyclic organic compounds containing n cross-conjugated exocyclic double bonds. The double bonds are commonly alkene groups but those with a carbonyl (C=O) group are also called radialenes. For some members the unsubstituted parent radialenes are elusive but many substituted derivatives are known.

Danishefsky's diene is an organosilicon compound and a diene with the formal name trans-1-methoxy-3-trimethylsilyloxy-buta-1,3-diene named after Samuel J. Danishefsky. Because the diene is very electron-rich it is a very reactive reagent in Diels-Alder reactions. This diene reacts rapidly with electrophilic alkenes, such as maleic anhydride. The methoxy group promotes highly regioselective additions. The diene is known to react with amines, aldehydes, alkenes and alkynes. Reactions with imines and nitro-olefins have been reported.
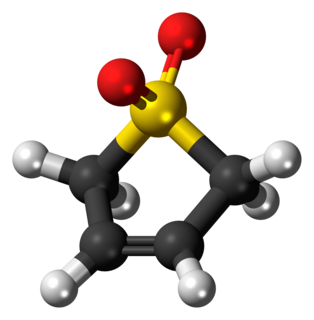
Sulfolene, or butadiene sulfone is a cyclic organic chemical with a sulfone functional group. It is a white, odorless, crystalline, indefinitely storable solid, which dissolves in water and many organic solvents. The compound is used as a source of butadiene.

Tetraphenylcyclopentadienone is an organic compound with the formula (C6H5)4C4CO. It is a dark purple to black crystalline solid that is soluble in organic solvents. It is an easily made building block for many organic and organometallic compounds.
In organic chemistry, an intramolecular Diels-Alder cycloaddition is a Diels–Alder reaction in which the diene and a dienophile are both part of the same molecule. The reaction leads to the formation of the same cyclohexene-like structure as usual for a Diels–Alder reaction, but as part of a more complex fused or bridged cyclic ring system. This reaction gives rise to various natural derivatives of decalin.
The retro-Diels–Alder reaction is the reverse of the Diels–Alder (DA) reaction, a [4+2] cycloelimination. It involves the formation of a diene and dienophile from a cyclohexene. It can be accomplished spontaneously with heat, or with acid or base mediation.
The imine Diels–Alder reaction involves the transformation of all-carbon dienes and imine dienophiles into tetrahydropyridines.

Torreyanic acid is a dimeric quinone first isolated and by Lee et al. in 1996 from an endophyte, Pestalotiopsis microspora. This endophyte is likely the cause of the decline of Florida torreya, an endangered species that is related to the taxol-producing Taxus brevifolia. The natural product was found to be cytotoxic against 25 different human cancer cell lines with an average IC50 value of 9.4 µg/mL, ranging from 3.5 (NEC) to 45 (A549) µg/mL. Torreyanic acid was found to be 5-10 times more potent in cell lines sensitive to protein kinase C (PKC) agonists, 12-o-tetradecanoyl phorbol-13-acetate (TPA), and was shown to cause cell death via apoptosis. Torreyanic acid also promoted G1 arrest of G0 synchronized cells at 1-5 µg/mL levels, depending on the cell line. It has been proposed that the eukaryotic translation initiation factor EIF-4a is a potential biochemical target for the natural compound.
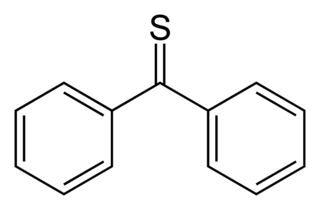
Thiobenzophenone is an organosulfur compound with the formula (C6H5)2CS. It is the prototypical thioketone. Unlike other thioketones that tend to dimerize to form rings and polymers, thiobenzophenone is quite stable, although it photoxidizes in air to form benzophenone and sulfur. Thiobenzophenone is deep blue and dissolves readily in many organic solvents.

Xylylene (sometimes quinone-dimethides) comprises two isomeric organic compounds with the formula C6H4(CH2)2. These compounds are related to the corresponding quinones and quinone methides by replacement of the oxygen atoms by CH2 groups. ortho- and para-xylylene are best known, although neither is stable in solid or liquid form. The meta form is a diradical. Certain substituted derivatives of xylylenes are however highly stable, an example being tetracyanoquinodimethane.
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes, between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.
The inverse electron demand Diels–Alder reaction, or DAINV or IEDDA is an organic chemical reaction, in which two new chemical bonds and a six-membered ring are formed. It is related to the Diels–Alder reaction, but unlike the Diels–Alder reaction, the DAINV is a cycloaddition between an electron-rich dienophile and an electron-poor diene. During a DAINV reaction, three pi-bonds are broken, and two sigma bonds and one new pi-bond are formed. A prototypical DAINV reaction is shown on the right.
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early and late d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen, nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.
A nitroalkene, or nitro olefin, is a functional group combining the functionality of its constituent parts, an alkene and nitro group, while displaying its own chemical properties through alkene activation, making the functional group useful in specialty reactions such as the Michael reaction or Diels-Alder additions.

1,3-Diphenylisobenzofuran is a highly reactive diene that can scavenge unstable and short-lived dienophiles in a Diels-Alder reaction. It is furthermore used as a standard reagent for the determination of singlet oxygen, even in biological systems. Cycloadditions with 1,3-diphenylisobenzofuran and subsequent oxygen cleavage provide access to a variety of polyaromatics.
Vinylcyclopropane [5+2] cycloaddition is a type of cycloaddition between a vinylcyclopropane (VCP) and an olefin or alkyne to form a seven-membered ring.