
Catharanthus roseus, commonly known as bright eyes, Cape periwinkle, graveyard plant, Madagascar periwinkle, old maid, pink periwinkle, rose periwinkle, is a perennial species of flowering plant in the family Apocynaceae. It is native and endemic to Madagascar, but is grown elsewhere as an ornamental and medicinal plant, and now has a pantropical distribution. It is a source of the drugs vincristine and vinblastine, used to treat cancer. It was formerly included in the genus Vinca as Vinca rosea.

Voacangine is an alkaloid found predominantly in the root bark of the Voacanga africana tree, as well as in other plants such as Tabernanthe iboga, Tabernaemontana africana, Trachelospermum jasminoides, Tabernaemontana divaricata and Ervatamia yunnanensis. It is an iboga alkaloid which commonly serves as a precursor for the semi-synthesis of ibogaine. It has been demonstrated in animals to have similar anti-addictive properties to ibogaine itself. It also potentiates the effects of barbiturates. Under UV-A and UV-B light its crystals fluoresce blue-green, and it is soluble in ethanol.
In enzymology, a secologanin synthase (EC 1.14.19.62, was wrongly classified as EC 1.3.3.9 in the past) is an enzyme that catalyzes the chemical reaction
In enzymology, a desacetoxyvindoline 4-hydroxylase (EC 1.14.11.20) is an enzyme that catalyzes the chemical reaction
In enzymology, a tabersonine 16-hydroxylase (EC 1.14.13.73) is an enzyme that catalyzes the chemical reaction
Strictosidine synthase (EC 4.3.3.2) is an enzyme in alkaloid biosynthesis that catalyses the condensation of tryptamine with secologanin to form strictosidine in a formal Pictet–Spengler reaction:

Ajmalicine, also known as δ-yohimbine or raubasine, is an antihypertensive drug used in the treatment of high blood pressure. It has been marketed under numerous brand names including Card-Lamuran, Circolene, Cristanyl, Duxil, Duxor, Hydroxysarpon, Iskedyl, Isosarpan, Isquebral, Lamuran, Melanex, Raunatin, Saltucin Co, Salvalion, and Sarpan. It is an alkaloid found naturally in various plants such as Rauvolfia spp., Catharanthus roseus, and Mitragyna speciosa.

Secologanin is a secoiridoid monoterpene synthesized from geranyl pyrophosphate in the mevalonate pathway. Secologanin then proceeds with dopamine or tryptamine to form ipecac and terpene indole alkaloids, respectively.
3α(S)-strictosidine β-glucosidase (EC 3.2.1.105) is an enzyme with systematic name strictosidine β-D-glucohydrolase. It catalyses the following chemical reaction:
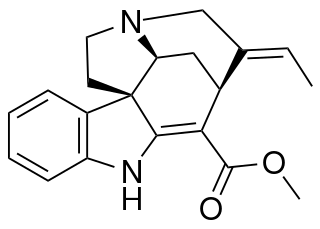
Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.

Strictosidine is a natural chemical compound and is classified as a glucoalkaloid and a vinca alkaloid. It is formed by the Pictet–Spengler condensation reaction of tryptamine with secologanin, catalyzed by the enzyme strictosidine synthase. Thousands of strictosidine derivatives are sometimes referred to by the broad phrase of monoterpene indole alkaloids. Strictosidine is an intermediate in the biosynthesis of numerous pharmaceutically valuable metabolites including quinine, camptothecin, ajmalicine, serpentine, vinblastine, vincristine and mitragynine.

Tabersonine is a terpene indole alkaloid found in the medicinal plant Catharanthus roseus and also in the genus Voacanga. Tabersonine is hydroxylated at the 16 position by the enzyme tabersonine 16-hydroxylase (T16H) to form 16-hydroxytabersonine. The enzyme leading to its formation is currently unknown. Tabersonine is the first intermediate leading to the formation of vindoline one of the two precursors required for vinblastine biosynthesis.

16-Hydroxytabersonine is a terpene indole alkaloid produced by the plant Catharanthus roseus. The metabolite is an intermediate in the formation of vindoline, a precursor needed for formation of the pharmaceutically valuable vinblastine and vincristine. 16-hydroxytabersonine is formed from the hydroxylation of tabersonine by tabersonine 16-hydroxylase (T16H). Tabersonine 16-O-methyltransferase (16OMT) methylates the hydroxylated 16 position to form 16-methoxytabersonine.

3-Hydroxy-16-methoxy-2,3-dihydrotabersonine is a terpene indole alkaloid produced by Catharanthus roseus. The metabolite is a substrate for 3-hydroxy-16-methoxy-2,3-dihydrotabersonine N-methyltransferase (NMT) which transfers a methyl group to the nitrogen of the indole ring forming desacetoxyvindoline. The enzyme catalyzing the formation of 3-hydroxy-16-methoxy-2,3-dihydrotabersonine from 16-methoxytabersonine is currently unknown, but is a result of hydration of the double bond connecting the 6 and 13 position carbons.

16-Methoxytabersonine is a terpene indole alkaloid produced by the medicinal plant Catharanthus roseus. 16-methoxytabersonine is synthesized by methylation of the hydroxyl group at the 16 position of 16-hydroxytabersonine by tabersonine 16-O-methyltransferase (16OMT). The compound is a substrate for hydration by two concerted enzymes Tabersonine-3-Oxidase (T3O) and Tabersonine-3-Reductase (T3R), which leads to the formation of 3-hydroxy-16-methoxy-2,3-dihydrotabersonine.

Deacetylvindoline is a terpene indole alkaloid produced by Catharanthus roseus. Deacetylvindoline is the product of a hydroxylation of desacetoxyvindoline by deacetoxyvindoline 4-hydroxylase (D4H). It is a substrate for deacetylvindoline O-acetyltransferase (DAT) which acetylates a hydroxy group to form vindoline, one of the two immediate precursors for the formation of the pharmacetucially valuable bisindole alkaloid vinblastine.

7-Deoxyloganic acid is an iridoid monoterpene. 7-Deoxyloganic acid is produced from 7-deoxyloganetic acid by the enzyme 7-deoxyloganetic acid glucosyltransferase (7-DLGT). The metabolite is a substrate for the enzyme 7-deoxyloganic acid hydroxylase (7-DLH) which synthesizes loganic acid.
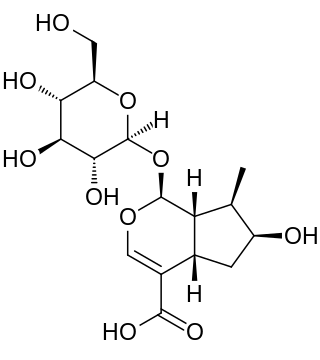
Loganic acid is an iridoid. Loganic acid is synthesized from 7-deoxyloganic acid by the enzyme 7-deoxyloganic acid hydroxylase (7-DLH). It is a substrate for the enzyme loganate O-methyltransferase for the production of loganin.
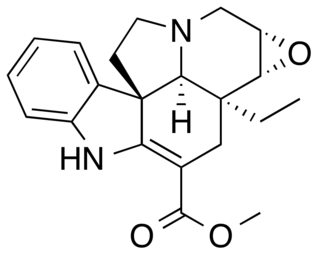
Lochnericine is a major monoterpene indole alkaloid present in the roots of Catharanthus roseus. It is also present in Tabernaemontana divaricata.

Catharanthine and vindoline are terpenoid indole alkaloids naturally produced within the Madagascar periwinkle plant whose dimerization produces the anti-cancer drugs vinblastine and vincristine. The precursor of catharanthine and vindoline is strictosidine, the common precursor of all indole alkaloids. The localization of catharanthine and vindoline within the plant tissue has been heavily studied in recent years with conflicting results. The dimerization of catharanthine and vindoline to form vinblastine and vincristine is catalyzed by a peroxidase and a reductase, and includes several intermediate compounds.















