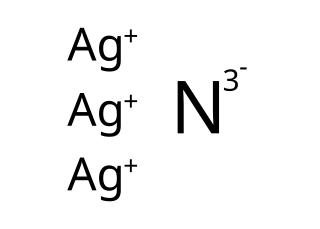An explosive is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances.

Mercury(II) fulminate, or Hg(CNO)2, is a primary explosive. It is highly sensitive to friction, heat and shock and is mainly used as a trigger for other explosives in percussion caps and detonators. Mercury(II) cyanate, though its chemical formula is identical, has a different atomic arrangement, making the cyanate and fulminate anionic isomers.

A detonator is a device used to make an explosive or explosive device explode. Detonators come in a variety of types, depending on how they are initiated and details of their inner working, which often involve several stages. Types of detonators include non-electric and electric. Non-electric detonators are typically stab or pyrotechnic while electric are typically "hot wire", exploding bridge wire or explosive foil.
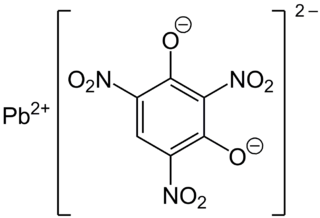
Lead styphnate (lead 2,4,6-trinitroresorcinate, C6HN3O8Pb ), whose name is derived from styphnic acid, is an explosive used as a component in primer and detonator mixtures for less sensitive secondary explosives. Lead styphnate is only slightly soluble in water and methanol. Samples of lead styphnate vary in color from yellow to gold, orange, reddish-brown, to brown. Lead styphnate is known in various polymorphs, hydrates, and basic salts. Normal lead styphnate monohydrate, monobasic lead styphnate, tribasic lead styphnate dihydrate, and pentabasic lead styphnate dehydrate as well as α, β polymorphs of lead styphnate exist.

A centre-fire is a type of metallic cartridge used in firearms, where the primer is located at the center of the base of its casing. Unlike rimfire cartridges, the centerfire primer is typically a separate component seated into a recessed cavity in the case head and is replaceable by reloading the cartridge.

Silver fulminate (AgCNO) is the highly explosive silver salt of fulminic acid.

Fulminic acid is an acid with the formula HCNO, more specifically H−C≡N+−O−. It is an isomer of isocyanic acid and of its elusive tautomer, cyanic acid, and also of isofulminic acid.
In organic chemistry, the diazo group is an organic moiety consisting of two linked nitrogen atoms at the terminal position. Overall charge-neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes and are described by the general structural formula R2C=N+=N−. The simplest example of a diazo compound is diazomethane, CH2N2. Diazo compounds should not be confused with azo compounds or with diazonium compounds.

Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, is a compound with the chemical formula HN3. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. The oxidation state of the nitrogen atoms in hydrazoic acid is fractional and is -1/3. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.
The Griess test is an analytical chemistry test which detects the presence of nitrite ion in solution. One of its most important uses is the determination of nitrite in drinking water. The Griess diazotization reaction, on which the Griess reagent relies, was first described in 1858 by Peter Griess. The test has also been widely used for the detection of nitrates, which are a common component of explosives, as they can be reduced to nitrites and detected with the Griess test.

Hexamethylene triperoxide diamine (HMTD) is a high explosive organic compound. HMTD is an organic peroxide, a heterocyclic compound with a cage-like structure. It is a primary explosive. It has been considered as an initiating explosive for blasting caps in the early part of 20th century, mostly because of its high initiating power and its inexpensive production. As such, it was quickly taken up as a primary explosive in mining applications. However, it has since been superseded by more (chemically) stable compounds such as dextrinated lead azide and DDNP. HMTD is widely used in amateur-made blasting caps.

Phenyl azide is an organic compound with the formula C6H5N3. It is one of the prototypical organic azides. It is a pale yellow oily liquid with a pungent odor. The structure consists of a linear azide substituent bound to a phenyl group. The C−N=N angle is approximately 120°. It was discovered in 1864 by Peter Griess by the reaction of ammonia and phenyldiazonium.

Johann Peter Griess was an industrial chemist and an early pioneer of organic chemistry. Griess was influential in the formation of modern dyes, first formulating the diazotization reaction of arylamines.

Potassium picrate, or potassium 2,4,6-trinitrophenolate, is an organic chemical, a picrate of potassium. It is a reddish yellow or green crystalline material. It is a primary explosive. Anhydrous potassium picrate forms orthorhombic crystals.

Lead(II) thiocyanate is a compound, more precisely a salt, with the formula Pb(SCN)2. It is a white crystalline solid, but will turn yellow upon exposure to light. It is slightly soluble in water and can be converted to a basic salt (Pb(CNS)2·Pb(OH)2 when boiled. Salt crystals may form upon cooling. Lead thiocyanate can cause lead poisoning if ingested and can adversely react with many substances. It has use in small explosives, matches, and dyeing.

Imidazole-1-sulfonyl azide is an organic azide compound that can be used as an alternative organic synthesis reagent to trifluoromethanesulfonyl azide. It is an explosive colorless liquid, but some of its organic-soluble salts can be safely handled and stored as a solid.
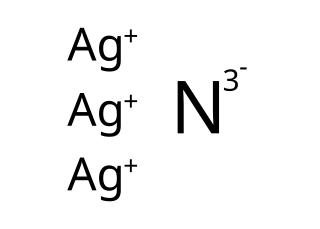
Silver nitride is an explosive chemical compound with symbol Ag3N. It is a black, metallic-looking solid which is formed when silver oxide or silver nitrate is dissolved in concentrated solutions of ammonia, causing formation of the diammine silver complex which subsequently breaks down to Ag3N. The standard free energy of the compound is about +315 kJ/mol, making it an endothermic compound which decomposes explosively to metallic silver and nitrogen gas.

Barium azide is an inorganic azide with the formula Ba(N3)2. It is a barium salt of hydrazoic acid. Like all azides, it is explosive. It is less sensitive to mechanical shock than lead azide.
In firearms and artillery, the primer is the chemical and/or device responsible for initiating the propellant combustion that will propel the projectiles out of the gun barrel.
An organic azide is an organic compound that contains an azide functional group. Because of the hazards associated with their use, few azides are used commercially although they exhibit interesting reactivity for researchers. Low molecular weight azides are considered especially hazardous and are avoided. In the research laboratory, azides are precursors to amines. They are also popular for their participation in the "click reaction" between an azide and an alkyne and in Staudinger ligation. These two reactions are generally quite reliable, lending themselves to combinatorial chemistry.