Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation with their odor.

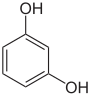
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (−OH) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, C
6H
5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.
In chemistry, a structural isomer of a compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct bonds between them. The term metamer was formerly used for the same concept.

In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring.
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.

In organic chemistry, a dicarbonyl is a molecule containing two carbonyl groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical or unsymmetrical.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.
Tetrahydropyran (THP) is the organic compound consisting of a saturated six-membered ring containing five carbon atoms and one oxygen atom. It is named by reference to pyran, which contains two double bonds, and may be produced from it by adding four hydrogens. In 2013, its preferred IUPAC name was established as oxane. The compound is a colourless volatile liquid. Derivatives of tetrahydropyran are, however, more common. 2-Tetrahydropyranyl (THP-) ethers derived from the reaction of alcohols and 3,4-dihydropyran are commonly used as protecting groups in organic synthesis. Furthermore, a tetrahydropyran ring system, i.e., five carbon atoms and an oxygen, is the core of pyranose sugars, such as glucose.

The Dakin oxidation (or Dakin reaction) is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde (2-hydroxybenzaldehyde or 4-hydroxybenzaldehyde) or ketone reacts with hydrogen peroxide (H2O2) in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidised, whereas the H2O2 is reduced.

In organic chemistry, dimethoxybenzene is an organic compound which is derived from benzene by substituting two methoxy groups. Dimethoxybenzene comes in three structural isomers:
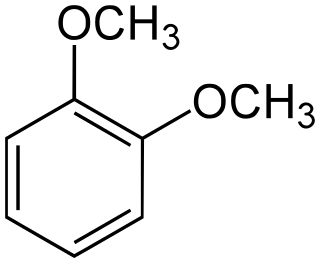
1,2-Dimethoxybenzene, commonly known as veratrole, is an organic compound with the formula C6H4(OCH3)2. It is one of three isomers of dimethoxybenzene. It is a colorless liquid, with a pleasant odor and slight solubility in water. It is the dimethyl ether derived from pyrocatechol.

1,2-Dichlorobenzene, or orthodichlorobenzene (ODCB), is an organic compound with the formula C6H4Cl2. This colourless liquid is poorly soluble in water but miscible with most organic solvents. It is a derivative of benzene, consisting of two adjacent chlorine atoms.

Divinylbenzene (DVB) is an organic compound with the chemical formula C6H4(CH=CH2)2 and structure H2C=CH−C6H4−HC=CH2. It is related to styrene by the addition of a second vinyl group. It is a colorless liquid manufactured by the thermal dehydrogenation of isomeric diethylbenzenes. Under synthesis conditions, o-divinylbenzene converts to naphthalene and thus is not a component of the usual mixtures of DVB.
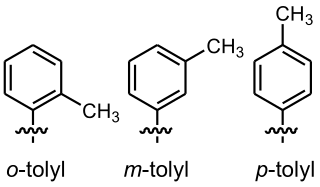
In organic chemistry, tolyl groups are functional groups related to toluene. They have the general formula CH3C6H4−R, the change of the relative position of the methyl and the R substituent on the aromatic ring can generate three possible structural isomers 1,2 (ortho), 1,3 (meta), and 1,4 (para). Tolyl groups are aryl groups which are commonly found in the structure of diverse chemical compounds. They are considered nonpolar and hydrophobic groups.
A hydroxynaphthoquinone is any of several organic compounds that can be viewed as derivatives of a naphthoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).
A hydroxybenzoquinone is any of several organic compounds that can be viewed as derivatives of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).

Hydroxy-1,4-benzoquinone, also called hydroxy-para-benzoquinone, is an organic compound with formula C
6H
4O
3, formally derived from 1,4-Benzoquinone by replacing one hydrogen atom with a hydroxyl (OH) group. It is one of three hydroxybenzoquinone isomers and one of the simplest hydroxyquinones.
A cyclohexanetetrol is a chemical compound consisting of a cyclohexane molecule with four hydroxyl groups (–OH) replacing four of the twelve hydrogen atoms. It is therefore a cyclitol. Its generic formula is C
6H
12O
4 or C
6H
8(OH)
4.

1,2,3,4-Cyclohexanetetrol (also named cyclohexane-1,2,3,4-tetrol, 1,2,3,4-tetrahydroxycyclohexane, or ortho-cyclohexanetetrol) is an organic compound whose molecule can be described as a cyclohexane with four hydroxyl (OH) groups substituted for hydrogen atoms on four consecutive carbon atoms. Its formula can be written C
6H
12O
4, C
6H
8(OH)
4, or (–CH(OH)–)4(–CH
2–)2.