In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them.

Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. They are white solids with the formula (C4H2S)n for the parent PT. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have substituents at the 3- or 4-position. They are also white solids, but tend to be soluble in organic solvents.

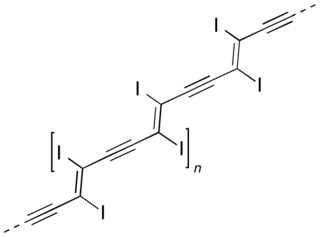
Polydiacetylenes (PDAs) are a family of conducting polymers closely related to polyacetylene. They are created by the 1,4 topochemical polymerization of diacetylenes. They have multiple applications from the development of organic films to immobilization of other molecules.

Dendrimers are repetitively branched molecules. The name comes from the Greek word δένδρον (dendron) which translates to "tree". Synonymous terms for dendrimer include arborols and cascade molecules. However, dendrimer is currently the internationally accepted term. A dendrimer is typically symmetric around the core, and often adopts a spherical three-dimensional morphology. The word dendron is also encountered frequently. A dendron usually contains a single chemically addressable group called the focal point or core. The difference between dendrons and dendrimers is illustrated in the top figure, but the terms are typically encountered interchangeably.
Methylaluminoxane, commonly called MAO, is an organoaluminium compound with the approximate formula (Al(CH3)O)n. Although it is usually encountered as a solution in (aromatic) solvents, commonly toluene but also xylene, cumene, or mesitylene, it can be isolated as a white pyrophoric solid. It is used to activate precatalysts for alkene polymerization.
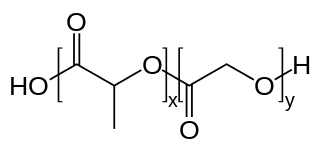
PLGA, PLG, or poly(lactic-co-glycolic acid) is a copolymer which is used in a host of Food and Drug Administration (FDA) approved therapeutic devices, owing to its biodegradability and biocompatibility. PLGA is synthesized by means of ring-opening co-polymerization of two different monomers, the cyclic dimers (1,4-dioxane-2,5-diones) of glycolic acid and lactic acid. Polymers can be synthesized as either random or block copolymers thereby imparting additional polymer properties. Common catalysts used in the preparation of this polymer include tin(II) 2-ethylhexanoate, tin(II) alkoxides, or aluminum isopropoxide. During polymerization, successive monomeric units are linked together in PLGA by ester linkages, thus yielding a linear, aliphatic polyester as a product.
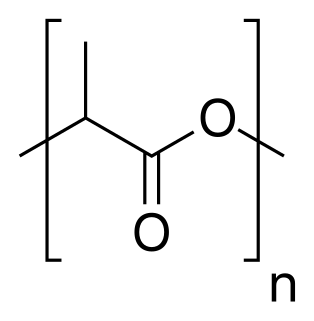
Polylactic acid or polylactide (PLA) is a thermoplastic aliphatic polyester derived from renewable biomass, typically from fermented plant starch such as from corn, cassava, sugarcane or sugar beet pulp. In 2010, PLA had the second highest consumption volume of any bioplastic of the world.
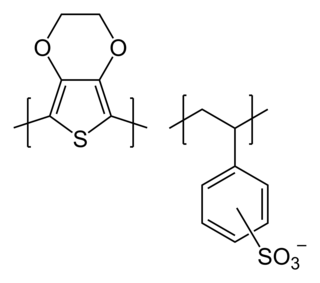
PEDOT:PSS or poly(3,4-ethylenedioxythiophene) polystyrene sulfonate is a polymer mixture of two ionomers. One component in this mixture is made up of sodium polystyrene sulfonate which is a sulfonated polystyrene. Part of the sulfonyl groups are deprotonated and carry a negative charge. The other component poly(3,4-ethylenedioxythiophene) or PEDOT is a conjugated polymer and carries positive charges and is based on polythiophene. Together the charged macromolecules form a macromolecular salt.
Polysulfones are a family of thermoplastic polymers. These polymers are known for their toughness and stability at high temperatures. They contain the subunit aryl-SO2-aryl, the defining feature of which is the sulfone group. Polysulfones were introduced in 1965 by Union Carbide. Due to the high cost of raw materials and processing, polysulfones are used in specialty applications and often are a superior replacement for polycarbonates.

Nanofibers are fibers with diameters in the nanometer range. Nanofibers can be generated from different polymers and hence have different physical properties and application potentials. Examples of natural polymers include collagen, cellulose, silk fibroin, keratin, gelatin and polysaccharides such as chitosan and alginate. Examples of synthetic polymers include poly(lactic acid) (PLA), polycaprolactone (PCL), polyurethane (PU), poly(lactic-co-glycolic acid) (PLGA), poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), and poly(ethylene-co-vinylacetate) (PEVA). Polymer chains are connected via covalent bonds. The diameters of nanofibers depend on the type of polymer used and the method of production. All polymer nanofibers are unique for their large surface area-to-volume ratio, high porosity, appreciable mechanical strength, and flexibility in functionalization compared to their microfiber counterparts.

Supramolecular polymers are a kind of polymers whose monomeric units hold together via highly directional and reversible non-covalent interactions. Unlike conventional bonded polymers, supramolecular polymers engage in a variety of non-covalent interactions that define their properties. These interactions include hydrogen bonding, π-π interaction, metal coordination, and host–guest interaction. Owing to the presence of these reversible noncovalent interactions, supramolecular polymers exhibit dynamic properties such as self-healing.
A halogen bond occurs when there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom in a molecular entity and a nucleophilic region in another, or the same, molecular entity.
Poly(N-isopropylacrylamide) is a temperature-responsive polymer that was first synthesized in the 1950s. It can be synthesized from N-isopropylacrylamide which is commercially available. It is synthesized via free-radical polymerization and is readily functionalized making it useful in a variety of applications.

Temperature-responsive polymers or thermoresponsive polymers are polymers that exhibit a drastic and discontinuous change of their physical properties with temperature. The term is commonly used when the property concerned is solubility in a given solvent, but it may also be used when other properties are affected. Thermoresponsive polymers belong to the class of stimuli-responsive materials, in contrast to temperature-sensitive materials, which change their properties continuously with environmental conditions. In a stricter sense, thermoresponsive polymers display a miscibility gap in their temperature-composition diagram. Depending on whether the miscibility gap is found at high or low temperatures, an upper or lower critical solution temperature exists, respectively.
The lower critical solution temperature (LCST) or lower consolute temperature is the critical temperature below which the components of a mixture are miscible for all compositions. The word lower indicates that the LCST is a lower bound to a temperature interval of partial miscibility, or miscibility for certain compositions only.

Polyethylenimine (PEI) or polyaziridine is a polymer with repeating unit composed of the amine group and two carbon aliphatic CH2CH2 spacer. Linear polyethyleneimines contain all secondary amines, in contrast to branched PEIs which contain primary, secondary and tertiary amino groups. Totally branched, dendrimeric forms were also reported. PEI is produced on industrial scale and finds many applications usually derived from its polycationic character.

Transparent conducting films (TCFs) are thin films of optically transparent and electrically conductive material. They are an important component in a number of electronic devices including liquid-crystal displays, OLEDs, touchscreens and photovoltaics. While indium tin oxide (ITO) is the most widely used, alternatives include wider-spectrum transparent conductive oxides (TCOs), conductive polymers, metal grids and random metallic networks, carbon nanotubes (CNT), graphene, nanowire meshes and ultra thin metal films.
In polymer physics, the coil–globule transition is the collapse of a macromolecule from an expanded coil state through an ideal coil state to a collapsed globule state, or vice versa. The coil–globule transition is of importance in biology due to the presence of coil-globule transitions in biological macromolecules such as proteins and DNA. It is also analogous with the swelling behavior of a crosslinked polymer gel and is thus of interest in biomedical engineering for controlled drug delivery. A particularly prominent example of a polymer possessing a coil-globule transition of interest in this area is that of Poly(N-isopropylacrylamide)(PNIPAAm).