
The haloalkanes are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.

A dimer is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. The term homodimer is used when the two molecules are identical and heterodimer when they are not. The reverse of dimerisation is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as Bjerrum pairs.
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is also much larger than the rate of chain propagation. The result is that the polymer chains grow at a more constant rate than seen in traditional chain polymerization and their lengths remain very similar. Living polymerization is a popular method for synthesizing block copolymers since the polymer can be synthesized in stages, each stage containing a different monomer. Additional advantages are predetermined molar mass and control over end-groups.
In polymer science, the backbone chain of a polymer is the longest series of covalently bonded atoms that together create the continuous chain of the molecule. This science is subdivided into the study of organic polymers, which consist of a carbon backbone, and inorganic polymers which have backbones containing only main group elements.

Organolithium reagents are organometallic compounds that contain carbon – lithium bonds. They are important reagents in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C-Li bond is highly ionic. Owing to the polar nature of the C-Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.

Conductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The biggest advantage of conductive polymers is their processability, mainly by dispersion. Conductive polymers are generally not thermoplastics, i.e., they are not thermoformable. But, like insulating polymers, they are organic materials. They can offer high electrical conductivity but do not show similar mechanical properties to other commercially available polymers. The electrical properties can be fine-tuned using the methods of organic synthesis and by advanced dispersion techniques.
Polyacetylene (IUPAC name: polyethyne) usually refers to an organic polymer with the repeating unit (C2H2)n. The name refers to its conceptual construction from polymerization of acetylene to give a chain with repeating olefin groups. This compound is conceptually important, as the discovery of polyacetylene and its high conductivity upon doping helped to launch the field of organic conductive polymers. The high electrical conductivity discovered by Hideki Shirakawa, Alan Heeger, and Alan MacDiarmid for this polymer led to intense interest in the use of organic compounds in microelectronics (organic semiconductors). This discovery was recognized by the Nobel Prize in Chemistry in 2000. Early work in the field of polyacetylene research was aimed at using doped polymers as easily processable and lightweight "plastic metals". Despite the promise of this polymer in the field of conductive polymers, many of its properties such as instability to air and difficulty with processing have led to avoidance in commercial applications.

Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. They are white solids with the formula (C4H2S)n for the parent PT. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have substituents at the 3- or 4-position. They are also white solids, but tend to be soluble in organic solvents.

Polydiacetylenes (PDAs) are a family of conducting polymers closely related to polyacetylene. They are created by the 1,4 topochemical polymerization of diacetylenes. They have multiple applications from the development of organic films to immobilization of other molecules.
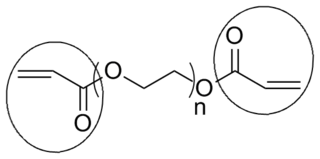
End groups are an important aspect of polymer synthesis and characterization. In polymer chemistry, end groups are functionalities or constitutional units that are at the extremity of a macromolecule or oligomer (IUPAC). In polymer synthesis, like condensation polymerization and free-radical types of polymerization, end-groups are commonly used and can be analyzed for example by nuclear magnetic resonance (NMR) to determine the average length of the polymer. Other methods for characterization of polymers where end-groups are used are mass spectrometry and vibrational spectrometry, like infrared and Raman spectrometry. Not only are these groups important for the analysis of the polymer, but they are also useful for grafting to and from a polymer chain to create a new copolymer. One example of an end group is in the polymer poly(ethylene glycol) diacrylate where the end-groups are circled.
Free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks. Free radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules. Following its generation, the initiating free radical adds (nonradical) monomer units, thereby growing the polymer chain.

In chemistry, a polyyne is any organic compound with alternating single and triple bonds; that is, a series of consecutive alkynes, (−C≡C−)
n with n greater than 1. The simplest example is diacetylene or butadiyne, H−C≡C−C≡C−H.
Atom transfer radical polymerization (ATRP) is an example of a reversible-deactivation radical polymerization. Like its counterpart, ATRA, or atom transfer radical addition, ATRP is a means of forming a carbon-carbon bond with a transition metal catalyst. The polymerization from this method is called atom transfer radical addition polymerization (ATRAP). As the name implies, the atom transfer step is crucial in the reaction responsible for uniform polymer chain growth. ATRP was independently discovered by Mitsuo Sawamoto and by Krzysztof Matyjaszewski and Jin-Shan Wang in 1995.
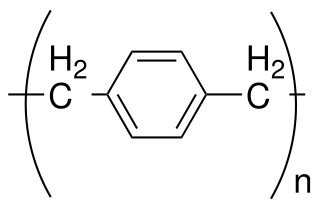
Parylene is the common name of a polymer whose backbone consists of para-benzenediyl rings –C
6H
4– connected by 1,2-ethanediyl bridges –CH
2–CH
2–. It can be obtained by polymerization of para-xylyleneH
2C=C
6H
4=CH
2.
A halogen bond occurs when there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom in a molecular entity and a nucleophilic region in another, or the same, molecular entity.
Living cationic polymerization is a living polymerization technique involving cationic propagating species. It enables the synthesis of very well defined polymers and of polymers with unusual architecture such as star polymers and block copolymers and living cationic polymerization is therefore as such of commercial and academic interest.
Living free radical polymerization is a type of living polymerization where the active polymer chain end is a free radical. Several methods exist. IUPAC recommends to use the term "reversible-deactivation radical polymerization" instead of "living free radical polymerization", though the two terms are not synonymous.
IUPAC Polymer Nomenclature are standardized naming conventions for polymers set by the International Union of Pure and Applied Chemistry (IUPAC) and described in their publication "Compendium of Polymer Terminology and Nomenclature", which is also known as the "Purple Book". Both the IUPAC and Chemical Abstracts Service (CAS) make similar naming recommendations for the naming of polymers.

Graft polymers are segmented copolymers with a linear backbone of one composite and randomly distributed branches of another composite. The picture labeled "graft polymer" shows how grafted chains of species B are covalently bonded to polymer species A. Although the side chains are structurally distinct from the main chain, the individual grafted chains may be homopolymers or copolymers. Graft polymers have been synthesized for many decades and are especially used as impact resistant materials, thermoplastic elastomers, compatibilizers, or emulsifiers for the preparation of stable blends or alloys. One of the more well known examples of a graft polymer is high impact polystyrene, which consists of a polystyrene backbone with polybutadiene grafted chains.

Diiodobutadiyne (1,4-diiodobuta-1,3-diyne) is a small molecule related to diacetylene. It is used in the creation of the polymer poly(diiododiacetylene) (PIDA) by undergoing 1,4 polymerization.












