Related Research Articles

Biochemistry, sometimes called biological chemistry, is the study of chemical processes within and relating to living organisms. Biochemical processes give rise to the complexity of life.

Enzymes are proteins that act as biological catalysts (biocatalysts). Catalysts accelerate chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called enzymology and a new field of pseudoenzyme analysis has recently grown up, recognising that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties.

Molecular biology is the branch of biology that concerns the molecular basis of biological activity in and between cells, including molecular synthesis, modification, mechanisms and interactions. The central dogma of molecular biology describes the process in which DNA is transcribed into RNA then translated into protein.

Superoxide dismutase (SOD, EC 1.15.1.1) is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide (O2−) radical into ordinary molecular oxygen (O2) and hydrogen peroxide (H2O2). Superoxide is produced as a by-product of oxygen metabolism and, if not regulated, causes many types of cell damage. Hydrogen peroxide is also damaging and is degraded by other enzymes such as catalase. Thus, SOD is an important antioxidant defense in nearly all living cells exposed to oxygen. One exception is Lactobacillus plantarum and related lactobacilli, which use a different mechanism to prevent damage from reactive O2−.

A superoxide is a compound that contains the superoxide ion, which has the chemical formula O−
2. The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature. Molecular oxygen (dioxygen) is a diradical containing two unpaired electrons, and superoxide results from the addition of an electron which fills one of the two degenerate molecular orbitals, leaving a charged ionic species with a single unpaired electron and a net negative charge of −1. Both dioxygen and the superoxide anion are free radicals that exhibit paramagnetism.

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins.
An oxidase is an enzyme that catalyzes an oxidation-reduction reaction, especially one involving dioxygen (O2) as the electron acceptor. In reactions involving donation of a hydrogen atom, oxygen is reduced to water (H2O) or hydrogen peroxide (H2O2). Some oxidation reactions, such as those involving monoamine oxidase or xanthine oxidase, typically do not involve free molecular oxygen.
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually utilizes NADP or NAD+ as cofactors. Transmembrane oxidoreductases create electron transport chains in bacteria, chloroplasts and mitochondria, including respiratory complexes I, II and III. Some others can associate with biological membranes as peripheral membrane proteins or be anchored to the membranes through a single transmembrane helix.

An oxidative enzyme is an enzyme that catalyses an oxidation reaction. Two most common types of oxidative enzymes are peroxidases, which use hydrogen peroxide, and oxidases, which use molecular oxygen. They increase the rate at which ATP is produced aerobically.
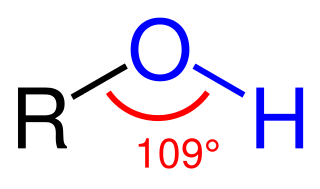
Alcohol oxidoreductases are oxidoreductase enzymes that act upon an alcohol functional group.
Clostripain is a proteinase that cleaves proteins on the carboxyl peptide bond of arginine. It was isolated from Clostridium histolyticum. The isoelectric point of the enzyme is 4.8-4.9, and optimum pH is 7.4~7.8. The composition of the enzyme is indicated to be of two chains of relative molecular mass 45,000 and 12,500.
Pyruvate dehydrogenase is an enzyme that catalyzes the reaction of pyruvate and a lipoamide to give the acetylated dihydrolipoamide and carbon dioxide. The conversion requires the coenzyme thiamine pyrophosphate.

Actinidain is a type of cysteine protease enzyme found in fruits including kiwifruit, pineapple, mango, banana and papaya. This enzyme is part of the papain-like peptidase C1 family.

Irwin Fridovich was an American biochemist who, together with his graduate student Joe M. McCord, discovered the enzymatic activity of copper-zinc superoxide dismutase (SOD),—to protect organisms from the toxic effects of superoxide free radicals formed as a byproduct of normal oxygen metabolism. Subsequently, Fridovich's research group also discovered the manganese-containing and the iron-containing SODs from E coli and the mitochondrial MnSOD (SOD2), now known to be an essential mammalian protein. He spent the rest of his career studying the biochemical mechanisms of SOD and of biological superoxide toxicity, using bacteria as model systems. Fridovich was also Professor Emeritus of Biochemistry at Duke University.
In enzymology, a formaldehyde dismutase is an enzyme that catalyzes the chemical reaction

Extracellular superoxide dismutase [Cu-Zn] is an enzyme that in humans is encoded by the SOD3 gene.
In enzymology, a trans-2-decenoyl-[acyl-carrier protein] isomerase is an enzyme that catalyzes the chemical reaction
In enzymology, a beta-ketoacyl-acyl-carrier-protein synthase I is an enzyme that catalyzes the chemical reaction

In enzymology and molecular biology, a holo-[acyl-carrier-protein] synthase is an enzyme that catalyzes the chemical reaction:

A diffusion-limited enzyme catalyses a reaction so efficiently that the rate limiting step is that of substrate diffusion into the active site, or product diffusion out. This is also known as kinetic perfection or catalytic perfection. Since the rate of catalysis of such enzymes is set by the diffusion-controlled reaction, it therefore represents an intrinsic, physical constraint on evolution. Diffusion limited perfect enzymes are very rare. Most enzymes catalyse their reactions to a rate that is 1,000-10,000 times slower than this limit. This is due to both the chemical limitations of difficult reactions, and the evolutionary limitations that such high reaction rates do not confer any extra fitness.
References
- ↑ Eric J. Toone (2006). Advances in Enzymology and Related Areas of Molecular Biology, Protein Evolution (Volume 75 ed.). Wiley-Interscience. ISBN 0471205036.
- ↑ Nicholas C. Price; Lewis Stevens (1999). Fundamentals of Enzymology: The Cell and Molecular Biology of Catalytic Proteins (Third ed.). USA: Oxford University Press. ISBN 019850229X.
- ↑ Kato N, Shirakawa K, Kobayashi H, Sakazawa C (1983). "The dismutation of aldehydes by a bacterial enzyme". Agric. Biol. Chem. 47: 39–46. doi: 10.1271/bbb1961.47.39 .
| This oxidoreductase article is a stub. You can help Wikipedia by expanding it. |