
The nitronium ion, [NO2]+, is a cation. It is an onium ion because its nitrogen atom has +1 charge, similar to ammonium ion [NH4]+. It is created by the removal of an electron from the paramagnetic nitrogen dioxide molecule NO2, or the protonation of nitric acid HNO3.
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2-dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates.

Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.

Cyanuric chloride is an organic compound with the formula (NCCl)3. This white solid is the chlorinated derivative of 1,3,5-triazine. It is the trimer of cyanogen chloride. Cyanuric chloride is the main precursor to the popular but controversial herbicide atrazine.

Tetrasulfur tetranitride is an inorganic compound with the formula S4N4. This vivid orange, opaque, crystalline explosive is the most important binary sulfur nitride, which are compounds that contain only the elements sulfur and nitrogen. It is a precursor to many S-N compounds and has attracted wide interest for its unusual structure and bonding.

A persistent carbene is an organic molecule whose natural resonance structure has a carbon atom with incomplete octet, but does not exhibit the tremendous instability typically associated with such moieties. The best-known examples and by far largest subgroup are the N-heterocyclic carbenes (NHC), in which nitrogen atoms flank the formal carbene.

Tetrathiafulvalene (TTF) is an organosulfur compound with the formula 2. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, (C5H4)2, by replacement of four CH groups with sulfur atoms. Over 10,000 scientific publications discuss TTF and its derivatives.

NanoPutians are a series of organic molecules whose structural formulae resemble human forms. James Tour's research group designed and synthesized these compounds in 2003 as a part of a sequence on chemical education for young students. The compounds consist of two benzene rings connected via a few carbon atoms as the body, four acetylene units each carrying an alkyl group at their ends which represents the hands and legs, and a 1,3-dioxolane ring as the head. Tour and his team at Rice University used the NanoPutians in their NanoKids educational outreach program. The goal of this program was to educate children in the sciences in an effective and enjoyable manner. They have made several videos featuring the NanoPutians as anthropomorphic animated characters.
In organic chemistry, the Hofmann–Löffler reaction (also referred to as Hofmann–Löffler–Freytag reaction, Löffler–Freytag reaction, Löffler–Hofmann reaction, as well as Löffler's method) is a cyclization reaction with remote C–H functionalization. In the reaction, thermal or photochemical decomposition of N-halogenated amine 1 in the presence of a strong acid (concentrated sulfuric acid or concentrated CF3CO2H) generates a nitrogen radical intermediate. The radical then abstracts an intramolecular hydrogen atom to give a cyclic amine 2 (pyrrolidine or, in some cases, piperidine).

The chemical compound 1,2-dioxetanedione, or 1,2-dioxacyclobutane-3,4-dione, often called peroxyacid ester, is an unstable oxide of carbon (an oxocarbon) with formula C2O4. It can be viewed as a double ketone of 1,2-dioxetane (1,2-dioxacyclobutane), or a cyclic dimer of carbon dioxide.
1,4,2-Dithiazole is a heterocyclic compound consisting of an unsaturated five-membered ring containing two carbon atoms, one nitrogen atom, and two sulfur atoms. 1,4,2-Dithiazole compounds may be formed by the reaction of nitrile sulfide with various reactive species; for instance thiocarbonyls via a 1,3-dipolar cycloaddition reaction. These compounds may be protonated by strong acids to give synthetically useful aromatic cations.

In chemistry, the term pseudo-oxocarbon anion is used to refer to a negative ion that is conceptually derived from an oxocarbon anion through replacement of one or more of the basic oxygen atoms by chemically similar elements or functional groups, such as sulfur (S), selenium (Se), or dicyanomethylene (=C(CN)2).
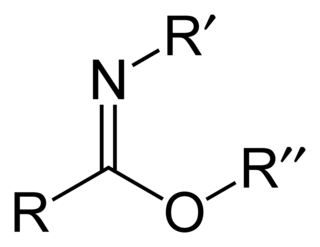
Carboximidates are organic compounds, which can be thought of as esters formed between a imidic acid and an alcohol, with the general formula R-C(=NR')OR".

Thianthrene is a sulfur-containing heterocyclic chemical compound. It is a derivative of the parent heterocycle called dithiin. It is notable for its ease of oxidation.
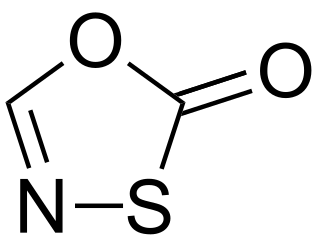
The oxathiazolones are a family of heterocyclic compounds in which the parent derivative has the molecular formula C2HNO2S and for which multiple isomers are known. The two known isomers with the highest profile in the literature are 1,3,4-oxathiazol-2-one and 1,4,2-oxathiazol-5-one.

Sulfoxylic acid (H2SO2) (also known as hyposulfurous acid or sulfur dihydroxide) is an unstable oxoacid of sulfur in an intermediate oxidation state between hydrogen sulfide and dithionous acid. It consists of two hydroxy groups attached to a sulfur atom. Sulfoxylic acid contains sulfur in an oxidation state of +2. Sulfur monoxide (SO) can be considered as a theoretical anhydride for sulfoxylic acid, but it is not actually known to react with water.

Trithiazyl trichloride is the inorganic compound with the formula (NSCl)3. A white solid, it is a precursor to other sulfur nitrides, but has no commercial applications.
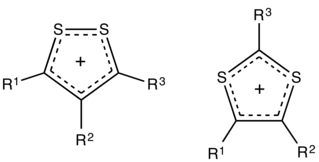
Dithiolium salts are compounds of the formula [(RC)3S2]+X− (R = H, alkyl, aryl, etc.). These salts consist of a planar organic cation with a variety of anions such as halides. The five-membered ring cations are observed in either of two isomers, 1,2- and 1,3-dithiolium cations. These cations differ with respect to the relative positions of the pair of sulfur atoms. Both isomers feature a planar ring, which is aromatic owing to the presence of 6π electrons. For example, the 1,2-ditholium ring can be represented as an allyl cation of the three carbons, with each sulfur atom donating one of its lone pairs of electrons to give a total of three pairs.

Dithionitronium hexafloroarsenate is the inorganic compound with the formula [SN2]AsF6. It is the hexafluoroarsenate (AsF−6) salt of S=N=S+. The cation is of interest as the sulfur analogue of nitronium (NO+2). Hexafloroarsenate is a weakly coordinating anion. According to X-ray crystallography, S=N=S+ is linear with S-N distances of 146 picometers.
Dithiazolium refers to families of heterocycles consisting of C2NS2 rings. The cations are aromatic on the grounds that they have six pi-electrons. In principle, several isomers are possible, depending on the relative location of the C, N, and S atoms in the ring.



















