Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation, dew, or fog to be present.
Coalbed methane extraction is a method for extracting methane from a coal deposit. Coal bed methane (CBM) is one of the factors restricting safe production of coal in underground coal mines. It is also a form of high-quality energy that can be used in many fields such as power generation, heating, and chemical industries. CBM extraction is therefore carried out prior to extraction with a view of increasing the safety of mining coal beds, and as a useful energy resource to be exploited.

Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate * on the surface of the adsorbent(solvent). This process differs from absorption, in which a fluid is dissolved by or permeates a liquid or solid. Adsorption is a surface phenomenon and does not penetrate through the surface to the bulk of the adsorbent, while absorption involves the whole volume of the material, although adsorption does often precede absorption. The term sorption encompasses both processes, while desorption is the reverse of it.

A dehumidifier is an air conditioning device which reduces and maintains the level of humidity in the air. This is done usually for health or thermal comfort reasons, or to eliminate musty odor and to prevent the growth of mildew by extracting water from the air. It can be used for household, commercial, or industrial applications. Large dehumidifiers are used in commercial buildings such as indoor ice rinks and swimming pools, as well as manufacturing plants or storage warehouses. Typical air conditioning systems combine dehumidification with cooling, by operating cooling coils below the dewpoint and draining away the water that condenses.
A desiccant is a hygroscopic substance that is used to induce or sustain a state of dryness (desiccation) in its vicinity; it is the opposite of a humectant. Commonly encountered pre-packaged desiccants are solids that absorb water. Desiccants for specialized purposes may be in forms other than solid, and may work through other principles, such as chemical bonding of water molecules. They are commonly encountered in foods to retain crispness. Industrially, desiccants are widely used to control the level of water in gas streams.

Psychrometrics is the field of engineering concerned with the physical and thermodynamic properties of gas-vapor mixtures.
Desorption is the physical process where a previously adsorbed substance is released from a surface. This happens when a molecule gains enough energy to overcome the activation barrier of the bounding energy that keeps it in the surface.

Water content or moisture content is the quantity of water contained in a material, such as soil, rock, ceramics, crops, or wood. Water content is used in a wide range of scientific and technical areas, and is expressed as a ratio, which can range from 0 to the value of the materials' porosity at saturation. It can be given on a volumetric or mass (gravimetric) basis.
Brunauer–Emmett–Teller (BET) theory aims to explain the physical adsorption of gas molecules on a solid surface and serves as the basis for an important analysis technique for the measurement of the specific surface area of materials. The observations are very often referred to as physical adsorption or physisorption. In 1938, Stephen Brunauer, Paul Hugh Emmett, and Edward Teller presented their theory in the Journal of the American Chemical Society. BET theory applies to systems of multilayer adsorption that usually utilizes a probing gas (called the adsorbate) that do not react chemically with the adsorptive (the material upon which the gas attaches to and the gas phase is called the adsorptive) to quantify specific surface area. Nitrogen is the most commonly employed gaseous adsorbate for probing surface(s). For this reason, standard BET analysis is most often conducted at the boiling temperature of N2 (77 K). Other probing adsorbates are also utilized, albeit less often, allowing the measurement of surface area at different temperatures and measurement scales. These include argon, carbon dioxide, and water. Specific surface area is a scale-dependent property, with no single true value of specific surface area definable, and thus quantities of specific surface area determined through BET theory may depend on the adsorbate molecule utilized and its adsorption cross section.
Moisture vapor transmission rate (MVTR), also water vapor transmission rate (WVTR), is a measure of the passage of water vapor through a substance. It is a measure of the permeability for vapor barriers.

Wood drying reduces the moisture content of wood before its use. When the drying is done in a kiln, the product is known as kiln-dried timber or lumber, whereas air drying is the more traditional method.

In chemical thermodynamics, isothermal titration calorimetry (ITC) is a physical technique used to determine the thermodynamic parameters of interactions in solution. It is most often used to study the binding of small molecules to larger macromolecules in a label-free environment. It consists of two cells which are enclosed in an adiabatic jacket. The compounds to be studied are placed in the sample cell, while the other cell, the reference cell, is used as a control and contains the buffer in which the sample is dissolved.
In physics and engineering, permeation is the penetration of a permeate through a solid. It is directly related to the concentration gradient of the permeate, a material's intrinsic permeability, and the materials' mass diffusivity. Permeation is modeled by equations such as Fick's laws of diffusion, and can be measured using tools such as a minipermeameter.

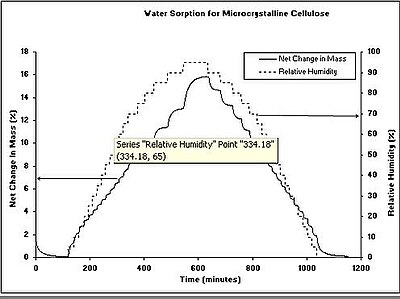
At equilibrium, the relationship between water content and equilibrium relative humidity of a material can be displayed graphically by a curve, the so-called moisture sorption isotherm. For each humidity value, a sorption isotherm indicates the corresponding water content value at a given, constant temperature. If the composition or quality of the material changes, then its sorption behaviour also changes. Because of the complexity of sorption process the isotherms cannot be determined explicitly by calculation, but must be recorded experimentally for each product.
Moisture analysis covers a variety of methods for measuring the moisture content in solids, liquids, or gases. For example, moisture is a common specification in commercial food production. There are many applications where trace moisture measurements are necessary for manufacturing and process quality assurance. Trace moisture in solids must be known in processes involving plastics, pharmaceuticals and heat treatment. Fields that require moisture measurement in gasses or liquids include hydrocarbon processing, pure semiconductor gases, bulk pure or mixed gases, dielectric gases such as those in transformers and power plants, and natural gas pipeline transport. Moisture content measurements can be reported in multiple units, such as: parts per million, pounds of water per million standard cubic feet of gas, mass of water vapor per unit volume or mass of water vapor per unit mass of dry gas.
Nanotube membranes are either a single, open-ended nanotube(CNT) or a film composed of an array of nanotubes that are oriented perpendicularly to the surface of an impermeable film matrix like the cells of a honeycomb. 'Impermeable' is essential here to distinguish nanotube membrane with traditional, well known porous membranes. Fluids and gas molecules may pass through the membrane en masse but only through the nanotubes. For instance, water molecules form ordered hydrogen bonds that act like chains as they pass through the CNTs. This results in an almost frictionless or atomically smooth interface between the nanotubes and water which relate to a "slip length" of the hydrophobic interface. Properties like the slip length that describe the non-continuum behavior of the water within the pore walls are disregarded in simple hydrodynamic systems and absent from the Hagen–Poiseuille equation. Molecular dynamic simulations better characterize the flow of water molecules through the carbon nanotubes with a varied form of the Hagen–Poiseuille equation that takes into account slip length.

Capillary condensation is the "process by which multilayer adsorption from the vapor [phase] into a porous medium proceeds to the point at which pore spaces become filled with condensed liquid from the vapor [phase]." The unique aspect of capillary condensation is that vapor condensation occurs below the saturation vapor pressure, Psat, of the pure liquid. This result is due to an increased number of van der Waals interactions between vapor phase molecules inside the confined space of a capillary. Once condensation has occurred, a meniscus immediately forms at the liquid-vapor interface which allows for equilibrium below the saturation vapor pressure. Meniscus formation is dependent on the surface tension of the liquid and the shape of the capillary, as shown by the Young-Laplace equation. As with any liquid-vapor interface involving a meniscus, the Kelvin equation provides a relation for the difference between the equilibrium vapor pressure and the saturation vapor pressure. A capillary does not necessarily have to be a tubular, closed shape, but can be any confined space with respect to its surroundings.
Inverse gas chromatography is a physical characterization analytical technique that is used in the analysis of the surfaces of solids.
The method of sorption calorimetry is designed for studies of hydration of complex organic and biological materials. It has been applied for studies of surfactants, lipids, DNA, nanomaterials and other substances. A sorption calorimetric experiment is performed at isothermal regime, but different temperatures can be studied in separate experiments.

Water activity (aw) is the partial vapor pressure of water in a solution divided by the standard state partial vapor pressure of water. In the field of food science, the standard state is most often defined as pure water at the same temperature. Using this particular definition, pure distilled water has a water activity of exactly one. Water activity is the thermodynamic activity of water as solvent and the relative humidity of the surrounding air after equilibration. As temperature increases, aw typically increases, except in some products with crystalline salt or sugar.