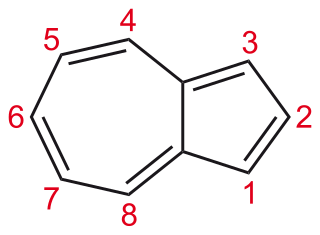
Azulene is an aromatic organic compound and an isomer of naphthalene. Naphthalene is colourless, whereas azulene is dark blue. The compound is named after its colour, as "azul" is Spanish for blue. Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that feature the azulene skeleton are found in nature as constituents of pigments in mushrooms, guaiac wood oil, and some marine invertebrates.
Total synthesis, a specialized area within organic chemistry, focuses on constructing complex organic compounds, especially those found in nature, using laboratory methods. It often involves synthesizing natural products from basic, commercially available starting materials. Total synthesis targets can also be organometallic or inorganic. While total synthesis aims for complete construction from simple starting materials, modifying or partially synthesizing these compounds is known as semisynthesis.
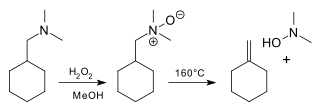
The Cope reaction or Cope elimination, developed by Arthur C. Cope, is the elimination reaction of an N-oxide to an alkene and a hydroxylamine.

Indazole, also called isoindazole, is a heterocyclic aromatic organic compound. This bicyclic compound consists of the fusion of benzene and pyrazole.
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the general subject of organic synthesis, there are many different types of synthetic routes that can be completed including total synthesis, stereoselective synthesis, automated synthesis, and many more. Additionally, in understanding organic synthesis it is necessary to be familiar with the methodology, techniques, and applications of the subject.
Tetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound with formula CH2N4, of which three isomers can be formulated.
The Hemetsberger indole synthesis is a chemical reaction that thermally decomposes a 3-aryl-2-azido-propenoic ester into an indole-2-carboxylic ester.
The Gabriel–Colman rearrangement is the chemical reaction of a saccharin or phthalimido ester with a strong base, such as an alkoxide, to form substituted isoquinolines. First described in 1900 by chemists Siegmund Gabriel and James Colman, this rearrangement, a ring expansion, is seen to be general if there is an enolizable hydrogen on the group attached to the nitrogen, since it is necessary for the nitrogen to abstract a hydrogen to form the carbanion that will close the ring. As shown in the case of the general example below, X is either CO or SO2.
The Wulff–Dötz reaction (also known as the Dötz reaction or the benzannulation reaction of the Fischer carbene complexes) is the chemical reaction of an aromatic or vinylic alkoxy pentacarbonyl chromium carbene complex with an alkyne and carbon monoxide to give a Cr(CO)3-coordinated substituted phenol. Several reviews have been published. It is named after the German chemist Karl Heinz Dötz (b. 1943) and the American chemist William D. Wulff (b. 1949) at Michigan State University. The reaction was first discovered by Karl Dötz and was extensively developed by his group and W. Wulff's group. They subsequently share the name of the reaction.
Robert S. Coleman is an American chemistry professor and researcher. Coleman was a faculty member at both Ohio State University and the University of South Carolina. At Ohio State, he was on the faculty in the Department of Chemistry from 1996 to 2012, having moved to Ohio State as an associate professor from the University of South Carolina. At USC, Coleman taught as assistant professor from 1989 to 1995, and then as associate professor from 1995 to 1996. In 1996, he accepted a faculty position at Ohio State University to teach Organic Chemistry, where he was an associate professor from 1996 until 2000. He was promoted to full professor in 2000, teaching Organic Chemistry up until his retirement in 2012. He received his Ph.D. degree working with Professor Dale L. Boger, completing the first total synthesis of the antitumor agent CC-1065. He was subsequently an NIH postdoctoral fellow at Yale University with Professor Samuel J. Danishefsky, where he completed, the first total synthesis of the aglycone of the antitumor agent calicheamicin.

The Neber rearrangement is an organic reaction in which a ketoxime is converted into an alpha-aminoketone via a rearrangement reaction.
Selenoxide elimination is a method for the chemical synthesis of alkenes from selenoxides. It is most commonly used to synthesize α,β-unsaturated carbonyl compounds from the corresponding saturated analogues. It is mechanistically related to the Cope reaction.
The Mislow–Evans rearrangement is a name reaction in organic chemistry. It is named after Kurt Mislow who reported the prototypical reaction in 1966, and David A. Evans who published further developments. The reaction allows the formation of allylic alcohols from allylic sulfoxides in a 2,3-sigmatropic rearrangement.
The Kharasch–Sosnovsky reaction is a method that involves using a copper or cobalt salt as a catalyst to oxidize olefins at the allylic position, subsequently condensing a peroxy ester or a peroxide resulting in the formation of allylic benzoates or alcohols via radical oxidation. This method is noteworthy for being the first allylic functionalization to utilize first-row transition metals and has found numerous applications in chemical and total synthesis. Chiral ligands can be used to render the reaction asymmetric, constructing chiral C–O bonds via C–H bond activation. This is notable as asymmetric addition to allylic groups tends to be difficult due to the transition state being highly symmetric. The reaction is named after Morris S. Kharasch and George Sosnovsky who first reported it in 1958. This method is noteworthy for being the first allylic functionalization to utilize first-row transition metals and has found numerous applications in chemical and total synthesis.
The Davis–Beirut reaction is N,N-bond forming heterocyclization that creates numerous types of 2H-indazoles and indazolones in both acidic and basic conditions The Davis–Beirut reaction is named after Mark Kurth and Makhluf Haddadin's respective universities; University of California, Davis and American University of Beirut, and is appealing because it uses inexpensive starting materials and does not require toxic metals.

Rick L. Danheiser is an American organic chemist and is the Arthur C. Cope Professor of Chemistry at the Massachusetts Institute of Technology and chair of the MIT faculty. His research involves the invention of new methods for the synthesis of complex organic compounds. Danheiser is known for the Danheiser annulation and Danheiser benzannulation reactions.
Vinylcyclopropane (5+2) cycloaddition is a type of cycloaddition between a vinylcyclopropane (VCP) and an olefin or alkyne to form a seven-membered ring.

Iodine azide is an explosive inorganic compound, which in ordinary conditions is a yellow solid. Formally, it is an inter-pseudohalogen.

The oxalate phosphates are chemical compounds containing oxalate and phosphate anions. They are also called oxalatophosphates or phosphate oxalates. Some oxalate-phosphate minerals found in bat guano deposits are known. Oxalate phosphates can form metal organic framework compounds.
Kathlyn Ann Parker is a chemist known for her work on synthesis of compounds, especially organic compounds with biological roles. She is an elected fellow of the American Chemical Society and a recipient of the Garvan–Olin Medal in chemistry.






