
Cellulase is any of several enzymes produced chiefly by fungi, bacteria, and protozoans that catalyze cellulolysis, the decomposition of cellulose and of some related polysaccharides:

An antifungal medication, also known as an antimycotic medication, is a pharmaceutical fungicide or fungistatic used to treat and prevent mycosis such as athlete's foot, ringworm, candidiasis (thrush), serious systemic infections such as cryptococcal meningitis, and others. Such drugs are usually obtained by a doctor's prescription, but a few are available over the counter (OTC). The evolution of antifungal resistance is a growing threat to health globally.

Aspergillus fumigatus is a species of fungus in the genus Aspergillus, and is one of the most common Aspergillus species to cause disease in individuals with an immunodeficiency.

Aspergillus is a genus consisting of several hundred mold species found in various climates worldwide.

Caspofungin is a lipopeptide antifungal drug from Merck & Co., Inc. discovered by James Balkovec, Regina Black and Frances A. Bouffard. It is a member of a new class of antifungals termed the echinocandins. It works by inhibiting the enzyme (1→3)-β-D-glucan synthase and thereby disturbing the integrity of the fungal cell wall. Caspofungin was the first inhibitor of fungal (1→3)-β-D-glucan synthesis to be approved by the United States Food and Drug Administration. Caspofungin is administered intravenously.

Anidulafungin (INN) is a semisynthetic echinocandin used as an antifungal drug. It was previously known as LY303366. It may also have application in treating invasive Aspergillus infection when used in combination with voriconazole. It is a member of the class of antifungal drugs known as the echinocandins; its mechanism of action is by inhibition of (1→3)-β-D-glucan synthase, an enzyme important to the synthesis of the fungal cell wall.
Pichia kudriavzevii is a budding yeast involved in chocolate production. P. kudriavzevii is an emerging fungal nosocomial pathogen primarily found in the immunocompromised and those with hematological malignancies. It has natural resistance to fluconazole, a standard antifungal agent. It is most often found in patients who have had prior fluconazole exposure, sparking debate and conflicting evidence as to whether fluconazole should be used prophylactically. Mortality due to P. kudriavzevii fungemia is much higher than the more common C. albicans. Other Candida species that also fit this profile are C. parapsilosis, C. glabrata, C. tropicalis, C. guillermondii and C. rugosa.

Aspergillus nidulans is one of many species of filamentous fungi in the phylum Ascomycota. It has been an important research organism for studying eukaryotic cell biology for over 50 years, being used to study a wide range of subjects including recombination, DNA repair, mutation, cell cycle control, tubulin, chromatin, nucleokinesis, pathogenesis, metabolism, and experimental evolution. It is one of the few species in its genus able to form sexual spores through meiosis, allowing crossing of strains in the laboratory. A. nidulans is a homothallic fungus, meaning it is able to self-fertilize and form fruiting bodies in the absence of a mating partner. It has septate hyphae with a woolly colony texture and white mycelia. The green colour of wild-type colonies is due to pigmentation of the spores, while mutations in the pigmentation pathway can produce other spore colours.
A glucan is a polysaccharide derived from D-glucose, linked by glycosidic bonds. Glucans are noted in two forms: alpha glucans and beta glucans. Many beta-glucans are medically important. They represent a drug target for antifungal medications of the echinocandin class.
Propionyl-CoA is a coenzyme A derivative of propionic acid. It is composed of a 24 total carbon chain and its production and metabolic fate depend on which organism it is present in. Several different pathways can lead to its production, such as through the catabolism of specific amino acids or the oxidation of odd-chain fatty acids. It later can be broken down by propionyl-CoA carboxylase or through the methylcitrate cycle. In different organisms, however, propionyl-CoA can be sequestered into controlled regions, to alleviate its potential toxicity through accumulation. Genetic deficiencies regarding the production and breakdown of propionyl-CoA also have great clinical and human significance.
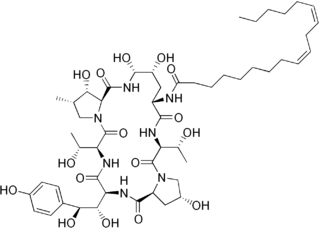
Echinocandins are a class of antifungal drugs that inhibit the synthesis of β-glucan in the fungal cell wall via noncompetitive inhibition of the enzyme 1,3-β glucan synthase. The class has been termed the "penicillin of antifungals," along with the related papulacandins, as their mechanism of action resembles that of penicillin in bacteria. β-glucans are carbohydrate polymers that are cross-linked with other fungal cell wall components, the fungal equivalent to bacterial peptidoglycan. Caspofungin, micafungin, and anidulafungin are semisynthetic echinocandin derivatives with limited clinical use due to their solubility, antifungal spectrum, and pharmacokinetic properties.
1,3-Beta-glucan synthase is a glucosyltransferase enzyme involved in the generation of beta-glucan in fungi. It serves as a pharmacological target for antifungal drugs such as caspofungin, anidulafungin, and micafungin, deemed 1,3-Beta-glucan synthase inhibitors. Under the CAZy classification system, fungi and plant members fall in the glycosyltransferase 48 family (GT48). Some members of the glycosyltransferase 2 family, such as the curdlan synthase CrdS, also has a similar activity.

Micafungin, sold under the brand name Mycamine, is an echinocandin antifungal medication used to treat and prevent invasive fungal infections including candidemia, abscesses, and esophageal candidiasis. It inhibits the production of beta-1,3-glucan, an essential component of fungal cell walls that is not found in mammals.
Chaetomium cupreum is a fungus in the family Chaetomiaceae. It is able to decay in manufactured cellulosic materials, and is known to antagonize a wide range of soil microorganisms. This species is component of the biocontrol agent, Ketomium, a commercial biofungicide. It has also been investigated for use in the production of natural dyes. Chaetomium cupreum is mesophilic and known to occur in harsh environments and can rapidly colonize organic substrates in soil. Laboratory cultures of C. cupreum can be propagated on a range of common growth media including potato dextrose at ambient or higher than ambient temperature producing cottony white colonies with a reddish reverse.
Glucan 1,3-α-glucosidase is an enzyme with systematic name 3-α-D-glucan 3-glucohydrolase. It catalyses the hydrolysis of terminal (1→3)-α-D-glucosidic links in (1→3)-α-D-glucans.
Cellulose 1,4-β-cellobiosidase is an enzyme of interest for its capability of converting cellulose to useful chemicals, particularly cellulosic ethanol.

Pneumocandin B0, also known as pneumocandin B0, pneumocandin B(0), and hydroxy echinocandin, is an organic chemical compound with the formula C50H80N8O17, produced by the fungus Glarea lozoyensis.

Édouard Georges Delacroix was a French mycologist and plant pathologist.

L-ornithine N5 monooxygenase (EC 1.14.13.195 or EC 1.14.13.196) is an enzyme which catalyzes one of the following chemical reactions:
L-ornithine + NADPH + O2 N(5)-hydroxy-L-ornithine + NADP+ + H2O L-ornithine + NAD(P)H + O2 N(5)-hydroxy-L-ornithine + NAD(P)+ + H2O
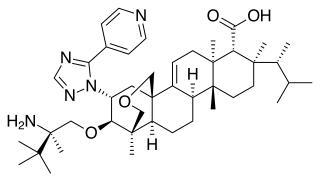
Ibrexafungerp, sold under the brand name Brexafemme, is an antifungal medication used to treat vulvovaginal candidiasis (VVC). It is taken orally. It is also currently undergoing clinical trials for other indications via an intravenous (IV) formulation. An estimated 75% of women will have at least one episode of VVC and 40 to 45% will have two or more episodes in their lifetime.














