Related Research Articles
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies. Immunotherapy is under preliminary research for its potential to treat various forms of cancer.
A cancer vaccine, or oncovaccine, is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.

Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology (immuno-oncology) and a growing subspecialty of oncology.

An antigen-presenting cell (APC) or accessory cell is a cell that displays an antigen bound by major histocompatibility complex (MHC) proteins on its surface; this process is known as antigen presentation. T cells may recognize these complexes using their T cell receptors (TCRs). APCs process antigens and present them to T cells.
Cross-presentation is the ability of certain professional antigen-presenting cells (mostly dendritic cells) to take up, process and present extracellular antigens with MHC class I molecules to CD8 T cells (cytotoxic T cells). Cross-priming, the result of this process, describes the stimulation of naive cytotoxic CD8+ T cells into activated cytotoxic CD8+ T cells. This process is necessary for immunity against most tumors and against viruses that infect dendritic cells and sabotage their presentation of virus antigens. Cross presentation is also required for the induction of cytotoxic immunity by vaccination with protein antigens, for example, tumour vaccination.

Ipilimumab, sold under the brand name Yervoy, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4, a protein receptor that downregulates the immune system.
Northwest Biotherapeutics, Inc. is a development-stage American pharmaceutical company headquartered in Maryland that focuses on developing immunotherapies against different types of cancer. It was founded in 1996 by Alton L. Boynton.
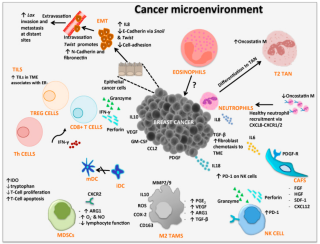
Cancer immunology (immuno-oncology) is an interdisciplinary branch of biology and a sub-discipline of immunology that is concerned with understanding the role of the immune system in the progression and development of cancer; the most well known application is cancer immunotherapy, which utilises the immune system as a treatment for cancer. Cancer immunosurveillance and immunoediting are based on protection against development of tumors in animal systems and (ii) identification of targets for immune recognition of human cancer.

Lymphocyte-activation gene 3, also known as LAG-3, is a protein which in humans is encoded by the LAG3 gene. LAG3, which was discovered in 1990 and was designated CD223 after the Seventh Human Leucocyte Differentiation Antigen Workshop in 2000, is a cell surface molecule with diverse biological effects on T cell function but overall has an immune inhibitory effect. It is an immune checkpoint receptor and as such is the target of various drug development programs by pharmaceutical companies seeking to develop new treatments for cancer and autoimmune disorders. In soluble form it is also being developed as a cancer drug in its own right.
Immunotransplant is a maneuver used to make vaccines more powerful. It refers to the process of infusing vaccine-primed T lymphocytes into lymphodepleted recipients for the purpose of enhancing the proliferation and function of those T cells and increasing immune protection induced by that vaccine.
Urelumab is a fully human, non‐ligand binding, CD137 agonist immunoglobulin‐γ 4 (IgG4) monoclonal antibody. It was developed utilizing Medarex's UltiMAb(R) technology by Bristol-Myers Squibb for the treatment of cancer and solid tumors. Urelumab promotes anti-tumor immunity, or an immune response against tumor cells, via CD137 activation. The application of Urelumab has been limited due to the fact that it can cause severe liver toxicity.
Myeloid-derived suppressor cells (MDSC) are a heterogeneous group of immune cells from the myeloid lineage.
PROSTVAC is a cancer immunotherapy candidate in clinical development by Bavarian Nordic for the treatment of all prostate cancer although clinical trials are focusing on more advanced cases of metastatic castration-resistant prostate cancer (mCRPC). PROSTVAC is a vaccine designed to enable the immune system to recognize and attack prostate cancer cells by triggering a specific and targeted T cell immune response to cancer cells that express the tumor-associated antigen prostate-specific antigen (PSA).

Immutep Ltd is a biotechnology company working primarily in the field of cancer immunotherapy using the LAG3 immune control mechanism. The company was originally built on CVac, a therapeutic cancer vaccine. In late 2014 the privately held French immunotherapy company Immutep SA was purchased by Prima Biotech.
Frédéric Triebel is a French immunologist who is best known for his 1990 discovery of the LAG3 immune control mechanism. Triebel worked through the 1990s in a collaboration between Institut Gustave Roussy and Merck Serono to establish LAG-3's mechanism of action in T cells and dendritic cells. In 2001 he founded Immutep SA, a biotech company, to develop the therapeutic potential of LAG3. In 2014 this company was acquired by Prima BioMed, where Triebel remains Chief Scientific and Medical Officer.
GSK2831781 is a monoclonal antibody being developed by GlaxoSmithKline (GSK) for autoimmune diseases. The antibody targets the T cell activation marker LAG-3, which is mainly expressed in inflamed tissues. In GSK's March 2015 Product development pipeline document the antibody is listed under 'Immuno-inflammation' candidates. GSK2831781 entered a Phase I clinical trial in psoriasis early in 2015.
The dendritic cell-based cancer vaccine is an innovation in therapeutic strategy for cancer patients.
Cancer vaccine targeting CD4+ T cells is a type of vaccine used to treat existing cancer. Cancerous cells usually cannot be recognized by the human immune system, and therefore cannot be destroyed. Some researchers state that cancer can be treated by increasing the response of T cells, especially CD4+ T cells, to cancerous cells through cancer vaccine injection.
APC Activators are a type of immunotherapy which leverages antigen-presenting cells (APCs) to drive an adaptive immune response. APC Activators are agonists to APC surface-expressed ligands that, when bound, induce the maturation and activation of APCs. Professional antigen-presenting cells – including dendritic cells, macrophages, and B cells – serve an indispensable role in the adaptive immune response through their unique ability to phagocytose, digest, and present exogenous (circulating) antigens to T cells, facilitating antigen-specific immune responses.
Whole-cell vaccines are a type of vaccine that has been prepared in the laboratory from entire cells. Such vaccines simultaneously contain multiple antigens to activate the immune system. They induce antigen-specific T-cell responses.
References
- ↑ Recommended INN: List 78 (PDF). Vol. 31. WHO Drug Information. 2017.
- ↑ Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T (May 1990). "LAG-3, a novel lymphocyte activation gene closely related to CD4". The Journal of Experimental Medicine. 171 (5): 1393–405. doi:10.1084/jem.171.5.1393. PMC 2187904 . PMID 1692078.
- ↑ Clinical trial number NCT00732082 for "Lag-3 and Gemcitabine for Treatment of Advanced Pancreas Cancer" at ClinicalTrials.gov
- ↑ Clinical trial number NCT00349934 for "IMP321 Plus First-line Paclitaxel in Metastatic Breast Carcinoma" at ClinicalTrials.gov
- ↑ Clinical trial number NCT00351949 for "IMP321 Phase 1 Trial in Metastatic Renal Cell Carcinoma (MRCC)" at ClinicalTrials.gov
- ↑ Clinical trial number NCT00354861 for "A Randomized Phase I Study of a Hepatitis B Antigen Combined With IMP321" at ClinicalTrials.gov
- ↑ Clinical trial number NCT00354263 for "Phase I Study of IMP321 Given Alone or as an Adjuvant to a Reference Flu Antigen" at ClinicalTrials.gov
- ↑ "Immutep and Eddingpharm sign agreement for development of ImmuFact IMP321 in China" (PDF) (Press release). October 8, 2013. Archived from the original (PDF) on February 13, 2015.