Related Research Articles

In bacteriology, gram-positive bacteria are bacteria that give a positive result in the Gram stain test, which is traditionally used to quickly classify bacteria into two broad categories according to their type of cell wall.
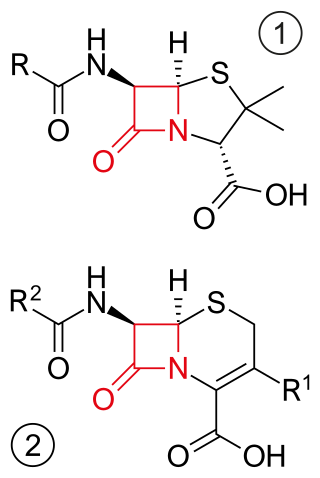
β-lactam antibiotics are antibiotics that contain a beta-lactam ring in their chemical structure. This includes penicillin derivatives (penams), cephalosporins and cephamycins (cephems), monobactams, carbapenems and carbacephems. Most β-lactam antibiotics work by inhibiting cell wall biosynthesis in the bacterial organism and are the most widely used group of antibiotics. Until 2003, when measured by sales, more than half of all commercially available antibiotics in use were β-lactam compounds. The first β-lactam antibiotic discovered, penicillin, was isolated from a strain of Penicillium rubens.

Vancomycin is a glycopeptide antibiotic medication used to treat a number of bacterial infections. It is used intravenously as a treatment for complicated skin infections, bloodstream infections, endocarditis, bone and joint infections, and meningitis caused by methicillin-resistant Staphylococcus aureus. Blood levels may be measured to determine the correct dose. Vancomycin is also taken orally as a treatment for severe Clostridium difficile colitis. When taken orally it is poorly absorbed.

Drug resistance is the reduction in effectiveness of a medication such as an antimicrobial or an antineoplastic in treating a disease or condition. The term is used in the context of resistance that pathogens or cancers have "acquired", that is, resistance has evolved. Antimicrobial resistance and antineoplastic resistance challenge clinical care and drive research. When an organism is resistant to more than one drug, it is said to be multidrug-resistant.
The cell envelope comprises the inner cell membrane and the cell wall of a bacterium. In gram-negative bacteria an outer membrane is also included. This envelope is not present in the Mollicutes where the cell wall is absent.

Glycopeptide antibiotics are a class of drugs of microbial origin that are composed of glycosylated cyclic or polycyclic nonribosomal peptides. Significant glycopeptide antibiotics include the anti-infective antibiotics vancomycin, teicoplanin, telavancin, ramoplanin and decaplanin, corbomycin, complestatin and the antitumor antibiotic bleomycin. Vancomycin is used if infection with methicillin-resistant Staphylococcus aureus (MRSA) is suspected.

Antibiotic sensitivity testing or antibiotic susceptibility testing is the measurement of the susceptibility of bacteria to antibiotics. It is used because bacteria may have resistance to some antibiotics. Sensitivity testing results can allow a clinician to change the choice of antibiotics from empiric therapy, which is when an antibiotic is selected based on clinical suspicion about the site of an infection and common causative bacteria, to directed therapy, in which the choice of antibiotic is based on knowledge of the organism and its sensitivities.

Penicillin-binding proteins (PBPs) are a group of proteins that are characterized by their affinity for and binding of penicillin. They are a normal constituent of many bacteria; the name just reflects the way by which the protein was discovered. All β-lactam antibiotics bind to PBPs, which are essential for bacterial cell wall synthesis. PBPs are members of a subgroup of enzymes called transpeptidases. Specifically, PBPs are DD-transpeptidases.
The rpoB gene encodes the β subunit of bacterial RNA polymerase and the homologous plastid-encoded RNA polymerase (PEP). It codes for 1342 amino acids in E. coli, making it the second-largest polypeptide in the bacterial cell. It is targeted by the rifamycin family of antibacterials, such as rifampin. Mutations in rpoB that confer resistance to rifamycins do so by altering the protein's drug-binding residues, thereby reducing affinity for these antibiotics.

Bacteria are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit soil, water, acidic hot springs, radioactive waste, and the deep biosphere of Earth's crust. Bacteria play a vital role in many stages of the nutrient cycle by recycling nutrients and the fixation of nitrogen from the atmosphere. The nutrient cycle includes the decomposition of dead bodies; bacteria are responsible for the putrefaction stage in this process. In the biological communities surrounding hydrothermal vents and cold seeps, extremophile bacteria provide the nutrients needed to sustain life by converting dissolved compounds, such as hydrogen sulphide and methane, to energy. Bacteria also live in symbiotic and parasitic relationships with plants and animals. Most bacteria have not been characterised and there are many species that cannot be grown in the laboratory. The study of bacteria is known as bacteriology, a branch of microbiology.

Dalbavancin, sold under the brand names Dalvance in the US and Xydalba in the EU among others, is a second-generation lipoglycopeptide antibiotic medication. It belongs to the same class as vancomycin, the most widely used and one of the treatments available to people infected with methicillin-resistant Staphylococcus aureus (MRSA).
Teixobactin is a peptide-like secondary metabolite of some species of bacteria, that kills some gram-positive bacteria. It appears to belong to a new class of antibiotics, and harms bacteria by binding to lipid II and lipid III, important precursor molecules for forming the cell wall.
NovoBiotic Pharmaceuticals is a privately held, early-stage biotechnology company focused on the discovery and development of new drugs from natural sources.

Lipid II is a precursor molecule in the synthesis of the cell wall of bacteria. It is a peptidoglycan, which is amphipathic and named for its bactoprenol hydrocarbon chain, which acts as a lipid anchor, embedding itself in the bacterial cell membrane. Lipid II must translocate across the cell membrane to deliver and incorporate its disaccharide-pentapeptide "building block" into the peptidoglycan mesh. Lipid II is the target of several antibiotics.
Mycoplasma orale is a small bacterium found in the class Mollicutes. It belongs to the genus Mycoplasma, a well-known group of bacterial parasites that inhabit humans. It also is known to be an opportunistic pathogen in immunocompromised humans. As with other Mycoplasma species, M. orale is not readily treated with many antibiotics due to its lack of a peptidoglycan cell wall. Therefore, this species is relevant to the medical field as physicians face the task of treating patients infected with this microbe. It is characterized by a small physical size, a small genome size, and a limited metabolism. It is also known to frequently contaminate laboratory experiments. This bacteria is very similar physiologically and morphologically to its sister species within the genus Mycoplasma; however, its recent discovery leaves many questions still unanswered about this microbe.
The Isolation chip is a method of culturing bacteria. Using regular methods, 99% of bacterial species are not able to be cultured as they do not grow in conditions made in a laboratory, a problem called the "Great Plate Count Anomaly". The ichip instead cultures bacterial species within its soil environment. The soil is diluted in molten agar and nutrients such that only a single cell, on average, grows in the ichip's small compartments or wells, hence the term "isolation". The chip is then enclosed in a semipermeable plastic membrane and buried back in the dirt to allow in nutrients not available in the lab. With this culturing method, about 50 to 60 percent of bacterial species are able to survive. Notably, the bacterial species Eleftheria terrae, which makes the antibiotic teixobactin that has shown promise against many drug-resistant strains like methicillin-resistant Staphylococcus aureus, was discovered using the ichip in 2015. In addition to antibiotics, it is argued that anti-cancer agents, anti-inflammatory and immunosuppressives as well as potential energy sources could be discovered. The ichip was developed by the drug discovery company NovoBiotic Pharmaceuticals, founded by Kim Lewis and Slava Epstein.
In microbiology, the term isolation refers to the separation of a strain from a natural, mixed population of living microbes, as present in the environment, for example in water or soil, or from living beings with skin flora, oral flora or gut flora, in order to identify the microbe(s) of interest. Historically, the laboratory techniques of isolation first developed in the field of bacteriology and parasitology, before those in virology during the 20th century.
Kim Lewis is an American researcher, author and academic. He is a University Distinguished Professor and the director of Antimicrobial Discovery Center at Northeastern University.
Slava Epstein is an American academic, researcher and entrepreneur working in the field of Microbial ecology. He is currently a professor in the biology department of Northeastern University and co-founder of NovoBiotic Pharmaceuticals. As a researcher his most covered contribution is the development of the Isolation chip (iChip) and the discovery of a new antibiotic, Teixobactin. Epstein's research has been published in many leading scientific journals including Nature and Science.
Undecaprenyl phosphate (UP), also known lipid-P, bactoprenol and C55-P., is a molecule with the primary function of trafficking polysaccharides across the cell membrane, largely contributing to the overall structure of the cell wall in Gram-positive bacteria. In some situations, UP can also be utilized to carry other cell-wall polysaccharides, but UP is the designated lipid carrier for peptidoglycan. During the process of carrying the peptidoglycan across the cell membrane, N-acetylglucosamine and N-acetylmuramic acid are linked to UP on the cytoplasmic side of the membrane before being carried across. UP works in a cycle of phosphorylation and dephosphorylation as the lipid carrier gets used, recycled, and reacts with undecaprenyl phosphate. This type of synthesis is referred to as de novo synthesis where a complex molecule is created from simpler molecules as opposed to a complete recycle of the entire structure.
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, Mueller A, Schäberle TF, Hughes DE, Epstein S, Jones M, Lazarides L, Steadman VA, Cohen DR, Felix CR, Fetterman KA, Millett WP, Nitti AG, Zullo AM, Chen C, Lewis K (7 January 2015). "A new antibiotic kills pathogens without detectable resistance". Nature . 517 (7535): 455–459. Bibcode:2015Natur.517..455L. doi:10.1038/nature14098. PMC 7414797 . PMID 25561178.
- ↑ Wright, Gerard (7 January 2015). "Antibiotics: An irresistible newcomer". Nature. 517 (7535): 442–444. Bibcode:2015Natur.517..442W. doi: 10.1038/nature14193 . PMID 25561172. S2CID 4464402.
- 1 2 3 4 5 Nichols, D.; Cahoon, N.; Trakhtenberg, E. M.; Pham, L.; Mehta, A.; Belanger, A.; Kanigan, T.; Lewis, K.; Epstein, S. S. (19 February 2010). "Use of Ichip for High-Throughput In Situ Cultivation of "Uncultivable" Microbial Species". Applied and Environmental Microbiology. 76 (8): 2445–2450. Bibcode:2010ApEnM..76.2445N. doi:10.1128/AEM.01754-09. PMC 2849220 . PMID 20173072.
- ↑ Servick, Kelly (7 January 2015). "Microbe found in grassy field contains powerful antibiotic". Science. doi:10.1126/science.aaa6305.
- ↑ Ledford, Heidi (7 January 2015). "Promising antibiotic discovered in microbial 'dark matter'". Nature. doi:10.1038/nature.2015.16675. S2CID 87719690.