
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general formula RR’C=N−OH, where R is an organic side-chain and R' may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure R1C(=NOH)NR2R3.

A dipeptide is an organic compound derived from two amino acids. The constituent amino acids can be the same or different. When different, two isomers of the dipeptide are possible, depending on the sequence. Several dipeptides are physiologically important, and some are both physiologically and commercially significant. A well known dipeptide is aspartame, an artificial sweetener.

In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis in living organisms occurs in the opposite direction.

N,N′-Dicyclohexylcarbodiimide (DCC or DCCD) is an organic compound with the chemical formula (C6H11N)2C. It is a waxy white solid with a sweet odor. Its primary use is to couple amino acids during artificial peptide synthesis. The low melting point of this material allows it to be melted for easy handling. It is highly soluble in dichloromethane, tetrahydrofuran, acetonitrile and dimethylformamide, but insoluble in water.

In organic chemistry, a carbodiimide is a functional group with the formula RN=C=NR. On Earth they are exclusively synthetic, but in interstellar space the parent compound HN=C=NH has been detected by its maser emissions.

1,1'-Carbonyldiimidazole (CDI) is an organic compound with the molecular formula (C3H3N2)2CO. It is a white crystalline solid. It is often used for the coupling of amino acids for peptide synthesis and as a reagent in organic synthesis.

Hydroxybenzotriazole is an organic compound that is a derivative of benzotriazole. It is a white crystalline powder, which as a commercial product contains some water. Anhydrous HOBt is explosive.

Pseudoproline derivatives are artificially created dipeptides to minimize aggregation during Fmoc solid-phase synthesis of peptides.

Bioconjugation is a chemical strategy to form a stable covalent link between two molecules, at least one of which is a biomolecule. Methods to conjugate biomolecules are applied in various field, including medicine, diagnostics, biocatalysis and materials. Synthetically modified biomolecules can have diverse functionalities, such as tracking cellular events, revealing enzyme function, determining protein biodistribution, imaging specific biomarkers, and delivering drugs to targeted cells.

N-Hydroxysuccinimide (NHS) is an organic compound with the formula (CH2CO)2NOH. It is a white solid that is used as a reagent for preparing active esters in peptide synthesis. It can be synthesized by heating succinic anhydride with hydroxylamine or hydroxylamine hydrochloride.

N,N′-Diisopropylcarbodiimide is a carbodiimide used in peptide synthesis. As a liquid, it is easier to handle than the commonly used N,N′-dicyclohexylcarbodiimide, a waxy solid. In addition, N,N′-diisopropylurea, its byproduct in many chemical reactions, is soluble in most organic solvents, a property that facilitates work-up.
The Fukuyama coupling is a coupling reaction taking place between a thioester and an organozinc halide in the presence of a palladium catalyst. The reaction product is a ketone. This reaction was discovered by Tohru Fukuyama et al. in 1998.

HATU is a reagent used in peptide coupling chemistry to generate an active ester from a carboxylic acid. HATU is used along with Hünig's base (N,N-diisopropylethylamine), or triethylamine to form amide bonds. Typically DMF is used as solvent, although other polar aprotic solvents can also be used.

1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide is a water-soluble carbodiimide usually handled as the hydrochloride.
2,5-Diketopiperazine is an organic compound with the formula (NHCH2C(O))2. The compound features a six-membered ring containing two amide groups at opposite positions in the ring. It was first compound containing a peptide bond to be characterized by X-ray crystallography in 1938. It is the parent of a large class of 2,5-Diketopiperazines (2,5-DKPs) with the formula (NHCH2(R)C(O))2 (R = H, CH3, etc.). They are ubiquitous peptide in nature. They are often found in fermentation broths and yeast cultures as well as embedded in larger more complex architectures in a variety of natural products as well as several drugs. In addition, they are often produced as degradation products of polypeptides, especially in processed foods and beverages. They have also been identified in the contents of comets.
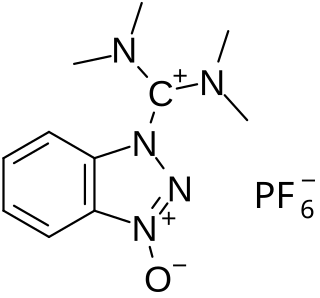
HBTU is a coupling reagent used in solid phase peptide synthesis. It was introduced in 1978 and shows resistance against racemization. It is used because of its mild activating properties.

PyAOP is a reagent used to prepare amides from carboxylic acids and amines in the context of peptide synthesis. It can be prepared from 1-hydroxy-7-azabenzotriazole (HOAt) and a chlorophosphonium reagent under basic conditions. It is a derivative of the HOAt family of amide bond forming reagents. It is preferred over HATU, because it does not engage in side reactions with the N-terminus of the peptide. Compared to the HOBt-containing analog PyBOP, PyAOP is more reactive due to the additional nitrogen in the fused pyridine ring of the HOAt moiety. Thermal hazard analysis by differential scanning calorimetry (DSC) shows PyAOP is potentially explosive.

N-Hydroxyphthalimide is the organic compound with the formula C6H4(CO)2NOH. A white or yellow solid, it is a derivative of phthalimide. The compound is as a catalyst in the synthesis of other organic compounds. It is soluble in water and organic solvents such as acetic acid, ethyl acetate and acetonitrile.

HCTU is an amidinium coupling reagent used in peptide synthesis. It is analogous to HBTU. The HOBt moiety has a chlorine in the 6 position which improves reaction rates and the synthesis of difficult couplings HCTU and related reagents containing the 6-chloro-1-hydroxybenzotriazole moiety can be prepared by reaction with TCFH under basic conditions. It can exist in an N-form (guanidinium) or an O-form (uronium), but the N-form is generally considered to be more stable for this class of reagent. In vivo dermal sensitization studies according to OECD 429 confirmed HCTU is a strong skin sensitizer, showing a response at 0.50 wt% in the Local Lymph Node Assay (LLNA) placing it in Globally Harmonized System of Classification and Labelling of Chemicals (GHS) Dermal Sensitization Category 1A.

In organic chemistry, an active ester is an ester functional group that is highly susceptible toward nucleophilic attack. Activation can be imparted by modifications of the acyl or the alkoxy components of a normal ester, say ethyl acetate. Typical modifications call for electronegative substituents. Active esters are employed in both synthetic and biological chemistry.






















