
Lymphoma is a group of blood and lymph tumors that develop from lymphocytes. The name typically refers to just the cancerous versions rather than all such tumours. Signs and symptoms may include enlarged lymph nodes, fever, drenching sweats, unintended weight loss, itching, and constantly feeling tired. The enlarged lymph nodes are usually painless. The sweats are most common at night.

Tumors of the hematopoietic and lymphoid tissues or tumours of the haematopoietic and lymphoid tissues are tumors that affect the blood, bone marrow, lymph, and lymphatic system. Because these tissues are all intimately connected through both the circulatory system and the immune system, a disease affecting one will often affect the others as well, making aplasia, myeloproliferation and lymphoproliferation closely related and often overlapping problems. While uncommon in solid tumors, chromosomal translocations are a common cause of these diseases. This commonly leads to a different approach in diagnosis and treatment of hematological malignancies. Hematological malignancies are malignant neoplasms ("cancer"), and they are generally treated by specialists in hematology and/or oncology. In some centers "hematology/oncology" is a single subspecialty of internal medicine while in others they are considered separate divisions. Not all hematological disorders are malignant ("cancerous"); these other blood conditions may also be managed by a hematologist.

Anaplastic large-cell lymphoma (ALCL) refers to a group of non-Hodgkin lymphomas in which aberrant T cells proliferate uncontrollably. Considered as a single entity, ALCL is the most common type of peripheral lymphoma and represents ~10% of all peripheral lymphomas in children. The incidence of ALCL is estimated to be 0.25 cases per 100,000 people in the United States of America. There are four distinct types of anaplastic large-cell lymphomas that on microscopic examination share certain key histopathological features and tumor marker proteins. However, the four types have very different clinical presentations, gene abnormalities, prognoses, and/or treatments.

Doxorubicin, sold under the brand name Adriamycin among others, is a chemotherapy medication used to treat cancer. This includes breast cancer, bladder cancer, Kaposi's sarcoma, lymphoma, and acute lymphocytic leukemia. It is often used together with other chemotherapy agents. Doxorubicin is given by injection into a vein.
CHOP is the acronym for a chemotherapy regimen used in the treatment of non-Hodgkin lymphoma. CHOP consists of:
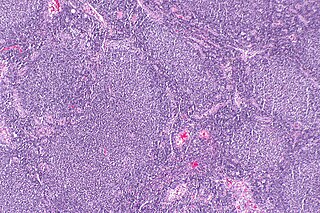
Follicular lymphoma (FL) is a cancer that involves certain types of white blood cells known as lymphocytes. The cancer originates from the uncontrolled division of specific types of B-cells known as centrocytes and centroblasts. These cells normally occupy the follicles in the germinal centers of lymphoid tissues such as lymph nodes. The cancerous cells in FL typically form follicular or follicle-like structures in the tissues they invade. These structures are usually the dominant histological feature of this cancer.
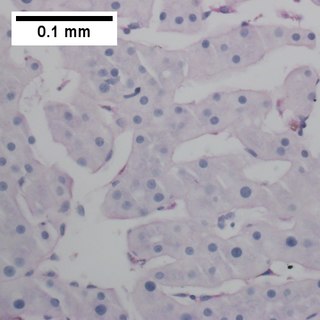
Primary effusion lymphoma (PEL) is classified as a diffuse large B cell lymphoma. It is a rare malignancy of plasmablastic cells that occurs in individuals that are infected with the Kaposi's sarcoma-associated herpesvirus. Plasmablasts are immature plasma cells, i.e. lymphocytes of the B-cell type that have differentiated into plasmablasts but because of their malignant nature do not differentiate into mature plasma cells but rather proliferate excessively and thereby cause life-threatening disease. In PEL, the proliferating plasmablastoid cells commonly accumulate within body cavities to produce effusions, primarily in the pleural, pericardial, or peritoneal cavities, without forming a contiguous tumor mass. In rare cases of these cavitary forms of PEL, the effusions develop in joints, the epidural space surrounding the brain and spinal cord, and underneath the capsule which forms around breast implants. Less frequently, individuals present with extracavitary primary effusion lymphomas, i.e., solid tumor masses not accompanied by effusions. The extracavitary tumors may develop in lymph nodes, bone, bone marrow, the gastrointestinal tract, skin, spleen, liver, lungs, central nervous system, testes, paranasal sinuses, muscle, and, rarely, inside the vasculature and sinuses of lymph nodes. As their disease progresses, however, individuals with the classical effusion-form of PEL may develop extracavitary tumors and individuals with extracavitary PEL may develop cavitary effusions.

T-cell lymphoma is a rare form of cancerous lymphoma affecting T-cells. Lymphoma arises mainly from the uncontrolled proliferation of T-cells and can become cancerous.
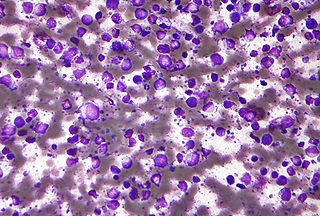
Diffuse large B-cell lymphoma (DLBCL) is a cancer of B cells, a type of lymphocyte that is responsible for producing antibodies. It is the most common form of non-Hodgkin lymphoma among adults, with an annual incidence of 7–8 cases per 100,000 people per year in the US and UK. This cancer occurs primarily in older individuals, with a median age of diagnosis at ~70 years, although it can occur in young adults and, in rare cases, children. DLBCL can arise in virtually any part of the body and, depending on various factors, is often a very aggressive malignancy. The first sign of this illness is typically the observation of a rapidly growing mass or tissue infiltration that is sometimes associated with systemic B symptoms, e.g. fever, weight loss, and night sweats.

Breast cancer chemotherapy refers to the use of cytotoxic drugs (chemotherapy) in the treatment of breast cancer.
Richter's transformation (RT), also known as Richter's syndrome, is the conversion of chronic lymphocytic leukemia (CLL) or its variant, small lymphocytic lymphoma (SLL), into a new and more aggressively malignant disease. CLL is the circulation of malignant B lymphocytes with or without the infiltration of these cells into lymphatic or other tissues while SLL is the infiltration of these malignant B lymphocytes into lymphatic and/or other tissues with little or no circulation of these cells in the blood. CLL along with its SLL variant are grouped together in the term CLL/SLL.

Aggressive lymphoma, also known as high-grade lymphoma, is a group of fast growing non-Hodgkin lymphoma.

Langerhans cell sarcoma (LCS) is a rare form of malignant histiocytosis. It should not be confused with Langerhans cell histiocytosis, which is cytologically benign. It can present most commonly in the skin and lymphatic tissue, but may also present in the lung, liver, and bone marrow. Treatment is most commonly with surgery or chemotherapy.
Lennert lymphoma, also termed lymphoepithelioid lymphoma, lymphoepithelioid variant of peripheral T-cell lymphoma, and epithelioid cellular lymphogranulomatosis, is a rare subtype of the T cell lymphomas. It was first characterized by Karl Lennert in 1952 as a variant of Hodgkin lymphoma based on the presence of cells resembling the Reed–Sternberg cells that typify Hodgkin lymphoma. However, later studies concluded that these cells are not Reed-Sternberg cells and that Lennert lymphoma is not a variant of Hodgkin lymphoma.

Extranodal NK/T-cell lymphoma, nasal type (ENKTCL-NT) is a rare type of lymphoma that commonly involves midline areas of the nasal cavity, oral cavity, and/or pharynx At these sites, the disease often takes the form of massive, necrotic, and extremely disfiguring lesions. However, ENKTCL-NT can also involve the eye, larynx, lung, gastrointestinal tract, skin, and various other tissues. ENKTCL-NT mainly affects adults; it is relatively common in Asia and to lesser extents Mexico, Central America, and South America but is rare in Europe and North America. In Korea, ENKTCL-NT often involves the skin and is reported to be the most common form of cutaneous lymphoma after mycosis fungoides.

Plasmablastic lymphoma (PBL) is a type of large B-cell lymphoma recognized by the World Health Organization (WHO) in 2017 as belonging to a subgroup of lymphomas termed lymphoid neoplasms with plasmablastic differentiation. The other lymphoid neoplasms within this subgroup are: plasmablastic plasma cell lymphoma ; primary effusion lymphoma that is Kaposi's sarcoma-associated herpesvirus positive or Kaposi's sarcoma-associated Herpesvirus negative; anaplastic lymphoma kinase-positive large B-cell lymphoma; and human herpesvirus 8-positive diffuse large B-cell lymphoma, not otherwise specified. All of these lymphomas are malignancies of plasmablasts, i.e. B-cells that have differentiated into plasmablasts but because of their malignant nature: fail to differentiate further into mature plasma cells; proliferate excessively; and accumulate in and injure various tissues and organs.
Epstein–Barr virus–associated lymphoproliferative diseases are a group of disorders in which one or more types of lymphoid cells, i.e. B cells, T cells, NK cells, and histiocytic-dendritic cells, are infected with the Epstein–Barr virus (EBV). This causes the infected cells to divide excessively, and is associated with the development of various non-cancerous, pre-cancerous, and cancerous lymphoproliferative disorders (LPDs). These LPDs include the well-known disorder occurring during the initial infection with the EBV, infectious mononucleosis, and the large number of subsequent disorders that may occur thereafter. The virus is usually involved in the development and/or progression of these LPDs although in some cases it may be an "innocent" bystander, i.e. present in, but not contributing to, the disease.
Primary testicular diffuse large B-cell lymphoma (PT-DLBCL), also termed testicular diffuse large B-cell lymphoma and diffuse large B-cell lymphoma of the testes, is a variant of the diffuse large B-cell lymphomas (DLBCL). DLBCL are a large and diverse group of B-cell malignancies with the great majority (-85%) being typed as diffuse large B-cell lymphoma, not otherwise specified. PT-DLBCL is a variant of DLBCL, NOS that involves one or, in uncommon cases, both testicles. Other variants and subtypes of DLBCL may involve the testes by spreading to them from their primary sites of origin in other tissues. PT-DLBCL differs from these other DLBCL in that it begins in the testes and then may spread to other sites.
Diffuse large B-cell lymphoma associated with chronic inflammation (DLBCL-CI) is a subtype of the Diffuse large B-cell lymphomas and a rare form of the Epstein–Barr virus-associated lymphoproliferative diseases, i.e. conditions in which lymphocytes infected with the Epstein-Barr virus (EBV) proliferate excessively in one or more tissues. EBV infects ~95% of the world's population to cause no symptoms, minor non-specific symptoms, or infectious mononucleosis. The virus then enters a latency phase in which the infected individual becomes a lifetime asymptomatic carrier of the virus. Some weeks, months, years, or decades thereafter, a very small fraction of these carriers, particularly those with an immunodeficiency, develop any one of various EBV-associated benign or malignant diseases.
Mature T-cell lymphoma, also called peripheral T-cell lymphoma, is a group of rare, aggressive lymphomas that develop from mature white blood cells and originate from lymphoid tissues outside of the bone marrow. Mature T-cell lymphoma is under the category of non-Hodgkin lymphoma. Mature T-cell lymphomas account for 10% to 15% of all lymphomas and is more common in Asia than in Europe and America. Its common subtypes include angioimmunoblastic T-cell lymphoma, anaplastic large cell lymphoma and peripheral T-cell lymphoma not otherwise specified. While different subtypes have variable symptoms, common symptoms include enlarged painless lymph nodes, fever, weight loss, rash and night sweats.

















