
dextro-Transposition of the great arteries is a potentially life-threatening birth defect in the large arteries of the heart. The primary arteries are transposed.

Atrial septal defect (ASD) is a congenital heart defect in which blood flows between the atria of the heart. Some flow is a normal condition both pre-birth and immediately post-birth via the foramen ovale; however, when this does not naturally close after birth it is referred to as a patent (open) foramen ovale (PFO). It is common in patients with a congenital atrial septal aneurysm (ASA).

The ostium primum atrial septal defect is a defect in the atrial septum at the level of the tricuspid and mitral valves. This is sometimes known as an endocardial cushion defect because it often involves the endocardial cushion, which is the portion of the heart where the atrial septum meets the ventricular septum and the mitral valve meets the tricuspid valve.

A congenital heart defect (CHD), also known as a congenital heart anomaly, congenital cardiovascular malformation, and congenital heart disease, is a defect in the structure of the heart or great vessels that is present at birth. A congenital heart defect is classed as a cardiovascular disease. Signs and symptoms depend on the specific type of defect. Symptoms can vary from none to life-threatening. When present, symptoms are variable and may include rapid breathing, bluish skin (cyanosis), poor weight gain, and feeling tired. CHD does not cause chest pain. Most congenital heart defects are not associated with other diseases. A complication of CHD is heart failure.

A ventricular septal defect (VSD) is a defect in the ventricular septum, the wall dividing the left and right ventricles of the heart. The extent of the opening may vary from pin size to complete absence of the ventricular septum, creating one common ventricle. The ventricular septum consists of an inferior muscular and superior membranous portion and is extensively innervated with conducting cardiomyocytes.

Cardiac catheterization is the insertion of a catheter into a chamber or vessel of the heart. This is done both for diagnostic and interventional purposes.

The atrium is one of the two upper chambers in the heart that receives blood from the circulatory system. The blood in the atria is pumped into the heart ventricles through the atrioventricular mitral and tricuspid heart valves.

Atrioventricular septal defect (AVSD) or atrioventricular canal defect (AVCD), also known as "common atrioventricular canal" or "endocardial cushion defect" (ECD), is characterized by a deficiency of the atrioventricular septum of the heart that creates connections between all four of its chambers. It is a very specific combination of 3 defects:

The fossa ovalis is a depression in the right atrium of the heart, at the level of the interatrial septum, the wall between right and left atrium. The fossa ovalis is the remnant of a thin fibrous sheet that covered the foramen ovale during fetal development.
In the fetal heart, the foramen ovale, also foramen Botalli or the ostium secundum of Born, allows blood to enter the left atrium from the right atrium. It is one of two fetal cardiac shunts, the other being the ductus arteriosus. Another similar adaptation in the fetus is the ductus venosus. In most individuals, the foramen ovale closes at birth. It later forms the fossa ovalis.

The interatrial septum is the wall of tissue that separates the right and left atria of the heart.

Balloon septostomy is the widening of a foramen ovale, patent foramen ovale (PFO), or atrial septal defect (ASD) via cardiac catheterization using a balloon catheter. This procedure allows a greater amount of oxygenated blood to enter the systemic circulation in some cases of cyanotic congenital heart defect (CHD).

During heart development of a human embryo, the single primitive atrium becomes divided into right and left by a septum, the septum primum. The septum primum grows downward into the single atrium.
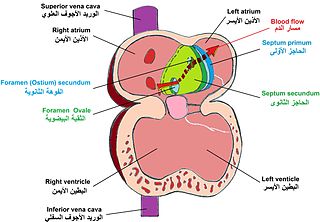
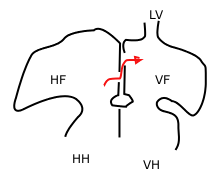
The septum secundum is a muscular flap that is important in heart development. It is semilunar in shape, and grows downward from the upper wall of the atrium immediately to the right of the septum primum and ostium secundum. It is important in the closure of the foramen ovale after birth.

In the developing heart, the atria are initially open to each other, with the opening known as the primary interatrial foramen or ostium primum. The foramen lies beneath the edge of septum primum and the endocardial cushions. It progressively decreases in size as the septum grows downwards, and disappears with the formation of the atrial septum.
The heart is the first functional organ in a vertebrate embryo. There are 5 stages to heart development.

Lutembacher's syndrome is a very rare form of congenital heart disease that affects one of the chambers of the heart as well as a valve. It is commonly known as both congenital atrial septal defect (ASD) and acquired mitral stenosis (MS). Congenital atrial septal defect refers to a hole being in the septum or wall that separates the two atria; this condition is usually seen in fetuses and infants. Mitral stenosis refers to mitral valve leaflets sticking to each other making the opening for blood to pass from the atrium to the ventricles very small. With the valve being so small, blood has difficulty passing from the left atrium into the left ventricle. Septal defects that may occur with Lutembacher's syndrome include: Ostium primum atrial septal defect or ostium secundum which is more prevalent.

Atrial septostomy is a surgical procedure in which a small hole is created between the upper two chambers of the heart, the atria. This procedure is primarily used to palliate dextro-Transposition of the great arteries or d-TGA, a life-threatening cyanotic congenital heart defect seen in infants. It is performed prior to an arterial switch operation. Atrial septostomy has also seen limited use as a surgical treatment for pulmonary hypertension. The first atrial septostomy was developed by Vivien Thomas in a canine model and performed in humans by Alfred Blalock. The Rashkind balloon procedure, a common atrial septostomy technique, was developed in 1966 by American cardiologist William Rashkind at the Children's Hospital of Philadelphia.

Left atrial appendage occlusion (LAAO), also referred to as left atrial appendage closure (LAAC), is a procedure used to reduce the risk of blood clots from the left atrial appendage entering the bloodstream and causing a stroke in those with non-valvular atrial fibrillation.

Heart development, also known as cardiogenesis, refers to the prenatal development of the heart. This begins with the formation of two endocardial tubes which merge to form the tubular heart, also called the primitive heart tube. The heart is the first functional organ in vertebrate embryos.
















