The visual cortex of the brain is the area of the cerebral cortex that processes visual information. It is located in the occipital lobe. Sensory input originating from the eyes travels through the lateral geniculate nucleus in the thalamus and then reaches the visual cortex. The area of the visual cortex that receives the sensory input from the lateral geniculate nucleus is the primary visual cortex, also known as visual area 1 (V1), Brodmann area 17, or the striate cortex. The extrastriate areas consist of visual areas 2, 3, 4, and 5.
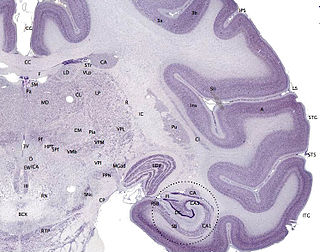
The cerebral cortex, also known as the cerebral mantle, is the outer layer of neural tissue of the cerebrum of the brain in humans and other mammals. The cerebral cortex mostly consists of the six-layered neocortex, with just 10% consisting of the allocortex. It is separated into two cortices, by the longitudinal fissure that divides the cerebrum into the left and right cerebral hemispheres. The two hemispheres are joined beneath the cortex by the corpus callosum. The cerebral cortex is the largest site of neural integration in the central nervous system. It plays a key role in attention, perception, awareness, thought, memory, language, and consciousness. The cerebral cortex is part of the brain responsible for cognition.

The basal ganglia (BG), or basal nuclei, are a group of subcortical nuclei found in the brains of vertebrates. In humans and some primates, differences exist, primarily in the division of the globus pallidus into external and internal regions, and in the division of the striatum. Positioned at the base of the forebrain and the top of the midbrain, they have strong connections with the cerebral cortex, thalamus, brainstem and other brain areas. The basal ganglia are associated with a variety of functions, including regulating voluntary motor movements, procedural learning, habit formation, conditional learning, eye movements, cognition, and emotion.
In biology, a reflex, or reflex action, is an involuntary, unplanned sequence or action and nearly instantaneous response to a stimulus.

The parietal lobe is one of the four major lobes of the cerebral cortex in the brain of mammals. The parietal lobe is positioned above the temporal lobe and behind the frontal lobe and central sulcus.

In neuroanatomy, the superior colliculus is a structure lying on the roof of the mammalian midbrain. In non-mammalian vertebrates, the homologous structure is known as the optic tectum, or optic lobe. The adjective form tectal is commonly used for both structures.

The motor cortex is the region of the cerebral cortex involved in the planning, control, and execution of voluntary movements. The motor cortex is an area of the frontal lobe located in the posterior precentral gyrus immediately anterior to the central sulcus.
Central pattern generators (CPGs) are self-organizing biological neural circuits that produce rhythmic outputs in the absence of rhythmic input. They are the source of the tightly-coupled patterns of neural activity that drive rhythmic and stereotyped motor behaviors like walking, swimming, breathing, or chewing. The ability to function without input from higher brain areas still requires modulatory inputs, and their outputs are not fixed. Flexibility in response to sensory input is a fundamental quality of CPG-driven behavior. To be classified as a rhythmic generator, a CPG requires:
- "two or more processes that interact such that each process sequentially increases and decreases, and
- that, as a result of this interaction, the system repeatedly returns to its starting condition."

Neural oscillations, or brainwaves, are rhythmic or repetitive patterns of neural activity in the central nervous system. Neural tissue can generate oscillatory activity in many ways, driven either by mechanisms within individual neurons or by interactions between neurons. In individual neurons, oscillations can appear either as oscillations in membrane potential or as rhythmic patterns of action potentials, which then produce oscillatory activation of post-synaptic neurons. At the level of neural ensembles, synchronized activity of large numbers of neurons can give rise to macroscopic oscillations, which can be observed in an electroencephalogram. Oscillatory activity in groups of neurons generally arises from feedback connections between the neurons that result in the synchronization of their firing patterns. The interaction between neurons can give rise to oscillations at a different frequency than the firing frequency of individual neurons. A well-known example of macroscopic neural oscillations is alpha activity.
A neuronal ensemble is a population of nervous system cells involved in a particular neural computation.
Head direction (HD) cells are neurons found in a number of brain regions that increase their firing rates above baseline levels only when the animal's head points in a specific direction. They have been reported in rats, monkeys, mice, chinchillas and bats, but are thought to be common to all mammals, perhaps all vertebrates and perhaps even some invertebrates, and to underlie the "sense of direction". When the animal's head is facing in the cell's "preferred firing direction" these neurons fire at a steady rate, but firing decreases back to baseline rates as the animal's head turns away from the preferred direction.

The scratch reflex is a response to activation of sensory neurons whose peripheral terminals are located on the surface of the body. Some sensory neurons can be activated by stimulation with an external object such as a parasite on the body surface. Alternatively, some sensory neurons can respond to a chemical stimulus that produces an itch sensation. During a scratch reflex, a nearby limb reaches toward and rubs against the site on the body surface that has been stimulated. The scratch reflex has been extensively studied to understand the functioning of neural networks in vertebrates. Despite decades of research, key aspects of the scratch reflex are still unknown, such as the neural mechanisms by which the reflex is terminated.
The zona incerta (ZI) is a horizontally elongated region of gray matter in the subthalamus below the thalamus. Its connections project extensively over the brain from the cerebral cortex down into the spinal cord.

The posterior parietal cortex plays an important role in planned movements, spatial reasoning, and attention.
Richard Alan Andersen is an American neuroscientist. He is the James G. Boswell Professor of Neuroscience at the California Institute of Technology in Pasadena, California. His research focuses on visual physiology with an emphasis on translational research to humans in the field of neuroprosthetics, brain-computer interfaces, and cortical repair.
The parietal reach region (PRR) is a region within the posterior parietal cortex of the macaque brain that is stimulated through reaching for an object. The PRR was initially proposed by Andersen and Buneo, but they just generally explored the idea as postural modulations. Dr. Steven Chang went further in depth by showing the receptive fields of PRR neurons are multiplicatively stimulated through the combination of initial gaze position and initial hand position. This multiplicative stimulation is known as gain-field encoding. The parietal reach region uses gain-field encoding to calculate the amount of hand displacement needed to reach for an object. A recent study shows that neurons in PRR encodes not only the planned physical movement, but also the anticipated visual sensory consequence of the intended movement once the action is unfolded in time.
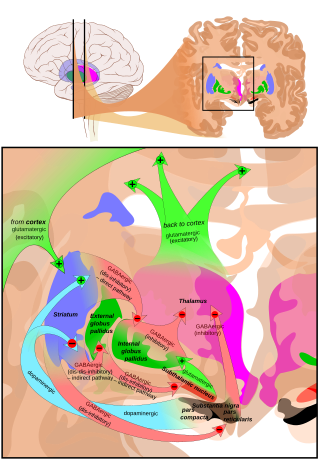
The cortico-basal ganglia-thalamo-cortical loop is a system of neural circuits in the brain. The loop involves connections between the cortex, the basal ganglia, the thalamus, and back to the cortex. It is of particular relevance to hyperkinetic and hypokinetic movement disorders, such as Parkinson's disease and Huntington's disease, as well as to mental disorders of control, such as attention deficit hyperactivity disorder (ADHD), obsessive–compulsive disorder (OCD), and Tourette syndrome.

Laura Busse is a German neuroscientist and professor of Systemic Neuroscience within the Division of Neurobiology at the Ludwig Maximilian University of Munich. Busse's lab studies context-dependent visual processing in mouse models by performing large scale in vivo electrophysiological recordings in the thalamic and cortical circuits of awake and behaving mice.

Eberhard Erich Fetz is an American neuroscientist, academic and researcher. He is a Professor of Physiology and Biophysics and DXARTS at the University of Washington.

Interlimb coordination is the coordination of the left and right limbs. It could be classified into two types of action: bimanual coordination and hands or feet coordination. Such coordination involves various parts of the nervous system and requires a sensory feedback mechanism for the neural control of the limbs. A model can be used to visualize the basic features, the control centre of locomotor movements, and the neural control of interlimb coordination. This coordination mechanism can be altered and adapted for better performance during locomotion in adults and for the development of motor skills in infants. The adaptive feature of interlimb coordination can also be applied to the treatment for CNS damage from stroke and the Parkinson's disease in the future.