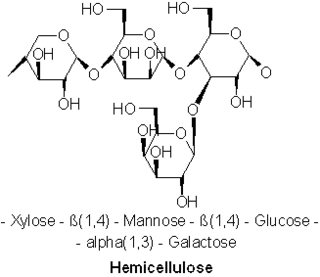
A hemicellulose is one of a number of heteropolymers, such as arabinoxylans, present along with cellulose in almost all terrestrial plant cell walls. Cellulose is crystalline, strong, and resistant to hydrolysis. Hemicelluloses are branched, shorter in length than cellulose, and also show a propensity to crystallize. They can be hydrolyzed by dilute acid or base as well as a myriad of hemicellulase enzymes.
Hydrolysis is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.

Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isobutyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, and beans and other legumes. It is encoded by the codons UUA, UUG, CUU, CUC, CUA, and CUG. Leucine is named after the Greek word for "white": λευκός (leukós, "white"), after its common appearance as a white powder, a property it shares with many other amino acids.

Cellulase is any of several enzymes produced chiefly by fungi, bacteria, and protozoans that catalyze cellulolysis, the decomposition of cellulose and of some related polysaccharides:
A glucoside is a glycoside that is chemically derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is hydrolysed by purely chemical means, or decomposed by fermentation or enzymes.

An exoenzyme, or extracellular enzyme, is an enzyme that is secreted by a cell and functions outside that cell. Exoenzymes are produced by both prokaryotic and eukaryotic cells and have been shown to be a crucial component of many biological processes. Most often these enzymes are involved in the breakdown of larger macromolecules. The breakdown of these larger macromolecules is critical for allowing their constituents to pass through the cell membrane and enter into the cell. For humans and other complex organisms, this process is best characterized by the digestive system which breaks down solid food via exoenzymes. The small molecules, generated by the exoenzyme activity, enter into cells and are utilized for various cellular functions. Bacteria and fungi also produce exoenzymes to digest nutrients in their environment, and these organisms can be used to conduct laboratory assays to identify the presence and function of such exoenzymes. Some pathogenic species also use exoenzymes as virulence factors to assist in the spread of these disease-causing microorganisms. In addition to the integral roles in biological systems, different classes of microbial exoenzymes have been used by humans since pre-historic times for such diverse purposes as food production, biofuels, textile production and in the paper industry. Another important role that microbial exoenzymes serve is in the natural ecology and bioremediation of terrestrial and marine environments.

Glucuronic acid is a uronic acid that was first isolated from urine. It is found in many gums such as gum arabic, xanthan, and kombucha tea and is important for the metabolism of microorganisms, plants and animals.

β-Hydroxy β-methylbutyric acid (HMB), otherwise known as its conjugate base, β-hydroxyβ-methylbutyrate, is a naturally produced substance in humans that is used as a dietary supplement and as an ingredient in certain medical foods that are intended to promote wound healing and provide nutritional support for people with muscle wasting due to cancer or HIV/AIDS. In healthy adults, supplementation with HMB has been shown to increase exercise-induced gains in muscle size, muscle strength, and lean body mass, reduce skeletal muscle damage from exercise, improve aerobic exercise performance, and expedite recovery from exercise. Medical reviews and meta-analyses indicate that HMB supplementation also helps to preserve or increase lean body mass and muscle strength in individuals experiencing age-related muscle loss. HMB produces these effects in part by stimulating the production of proteins and inhibiting the breakdown of proteins in muscle tissue. No adverse effects from long-term use as a dietary supplement in adults have been found.

Ferulic acid is a hydroxycinnamic acid derivative and a phenolic compound. It is an organic compound with the formula (CH3O)HOC6H3CH=CHCO2H. The name is derived from the genus Ferula, referring to the giant fennel (Ferula communis). Classified as a phenolic phytochemical, ferulic acid is an amber colored solid. Esters of ferulic acid are found in plant cell walls, covalently bonded to hemicellulose such as arabinoxylans. Salts and esters derived from ferulic acid are called ferulates.

Isovaleric acid, also known as 3-methylbutanoic acid or β-methylbutyric acid, is a branched-chain alkyl carboxylic acid with the chemical formula (CH3)2CHCH2CO2H. It is classified as a short-chain fatty acid. Like other low-molecular-weight carboxylic acids, it has an unpleasant odor. The compound occurs naturally and can be found in many foods, such as cheese, soy milk, and apple juice.

β-Glucosidase is an enzyme that catalyses the following reaction:

β-Hydroxybutyric acid, also known as 3-hydroxybutyric acid or BHB, is an organic compound and a beta hydroxy acid with the chemical formula CH3CH(OH)CH2CO2H; its conjugate base is β-hydroxybutyrate, also known as 3-hydroxybutyrate. β-Hydroxybutyric acid is a chiral compound with two enantiomers: D-β-hydroxybutyric acid and L-β-hydroxybutyric acid. Its oxidized and polymeric derivatives occur widely in nature. In humans, D-β-hydroxybutyric acid is one of two primary endogenous agonists of hydroxycarboxylic acid receptor 2 (HCA2), a Gi/o-coupled G protein-coupled receptor (GPCR).
Methylcrotonyl CoA carboxylase is a biotin-requiring enzyme located in the mitochondria. MCC uses bicarbonate as a carboxyl group source to catalyze the carboxylation of a carbon adjacent to a carbonyl group performing the fourth step in processing leucine, an essential amino acid.

Mannose-6 phosphate isomerase (MPI), alternately phosphomannose isomerase (PMI) is an enzyme which facilitates the interconversion of fructose 6-phosphate (F6P) and mannose-6-phosphate (M6P). Mannose-6-phosphate isomerase may also enable the synthesis of GDP-mannose in eukaryotic organisms. M6P can be converted to F6P by mannose-6-phosphate isomerase and subsequently utilized in several metabolic pathways including glycolysis and capsular polysaccharide biosynthesis. PMI is monomeric and metallodependent on zinc as a cofactor ligand. PMI is inhibited by erythrose 4-phosphate, mannitol 1-phosphate, and to a lesser extent, the alpha anomer of M6P.

In enzymology, an isovaleryl-CoA dehydrogenase is an enzyme that catalyzes the chemical reaction
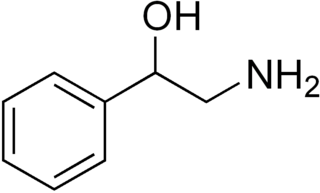
Phenylethanolamine, or β-hydroxyphenethylamine, is a trace amine with a structure similar to those of other trace phenethylamines as well as the catecholamine neurotransmitters dopamine, norepinephrine, and epinephrine. As an organic compound, phenylethanolamine is a β-hydroxylated phenethylamine that is also structurally related to a number of synthetic drugs in the substituted phenethylamine class. In common with these compounds, phenylethanolamine has strong cardiovascular activity and, under the name Apophedrin, has been used as a drug to produce topical vasoconstriction.

In winemaking, clarification and stabilization are the processes by which insoluble matter suspended in the wine is removed before bottling. This matter may include dead yeast cells (lees), bacteria, tartrates, proteins, pectins, various tannins and other phenolic compounds, as well as pieces of grape skin, pulp, stems and gums. Clarification and stabilization may involve fining, filtration, centrifugation, flotation, refrigeration, pasteurization, and/or barrel maturation and racking.

1,2,3,4,6-Pentagalloylglucose is the pentagallic acid ester of glucose. It is a gallotannin and the precursor of ellagitannins.
4-Hydroxy-7-methoxy-3-oxo-3,4-dihydro-2H-1,4-benzoxazin-2-yl glucoside beta-D-glucosidase (EC 3.2.1.182, DIMBOAGlc hydrolase, DIMBOA glucosidase) is an enzyme with systematic name (2R)-4-hydroxy-7-methoxy-3-oxo-3,4-dihydro-2H-1,4-benzoxazin-2-yl beta-D-glucopyranoside beta-D-glucosidase. This enzyme catalyses the following chemical reaction

β-Hydroxy β-methylbutyryl-coenzyme A (HMB-CoA), also known as 3-hydroxyisovaleryl-CoA, is a metabolite of L-leucine that is produced in the human body. Its immediate precursors are β-hydroxy β-methylbutyric acid (HMB) and β-methylcrotonoyl-CoA (MC-CoA). It can be metabolized into HMB, MC-CoA, and HMG-CoA in humans.















