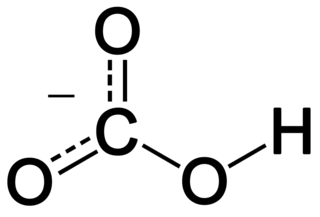
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula HCO−
3.

Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the Pauling scale, behind only oxygen and fluorine.

A carboxylic acid is an organic compound that contains a carboxyl group. The general formula of a carboxylic acid is R–COOH, with R referring to the alkyl group. Carboxylic acids occur widely. Important examples include the amino acids and acetic acid. Deprotonation of a carboxyl group gives a carboxylate anion.
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed by the reception of a proton (H+) by a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a hydrogen ion. On the other hand, a conjugate base is what is left over after an acid has donated a proton during a chemical reaction. Hence, a conjugate base is a species formed by the removal of a proton from an acid, as in the reverse reaction it is able to gain a hydrogen ion. Because some acids are capable of releasing multiple protons, the conjugate base of an acid may itself be acidic.
An anaerobic organism or anaerobe is any organism that does not require oxygen for growth. It may react negatively or even die if free oxygen is present. In contrast, an aerobic organism (aerobe) is an organism that requires an oxygenated environment. Anaerobes may be unicellular or multicellular.

In chemistry, bases are substances that, in aqueous solution, release hydroxide (OH−) ions, are slippery to the touch, can taste bitter if an alkali, change the color of indicators (e.g., turn red litmus paper blue), react with acids to form salts, promote certain chemical reactions (base catalysis), accept protons from any proton donor or contain completely or partially displaceable OH− ions. Examples of bases are the hydroxides of the alkali metals and the alkaline earth metals (NaOH, Ca(OH)2, etc.—see alkali hydroxide and alkaline earth hydroxide).

Hydrogen sulfide is the chemical compound with the formula H
2S. It is a colorless chalcogen hydride gas with the characteristic foul odor of rotten eggs. It is very poisonous, corrosive, and flammable.

Sodium bicarbonate (IUPAC name: sodium hydrogen carbonate), commonly known as baking soda (especially in North America and New Zealand) or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation (Na+) and a bicarbonate anion (HCO3−). Sodium bicarbonate is a white solid that is crystalline, but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda (sodium carbonate). The natural mineral form is nahcolite. It is a component of the mineral natron and is found dissolved in many mineral springs.

Sodium carbonate, Na2CO3, (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, water-soluble salts. All forms have a strongly alkaline taste and give moderately alkaline solutions in water. Historically it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process.
Reducing agent is an element or compound that loses an electron to an electron recipient in a redox chemical reaction.
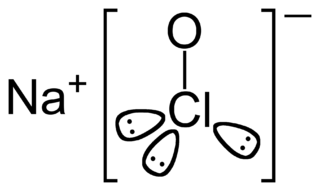
Sodium hypochlorite is a chemical compound with the formula NaOCl or NaClO, comprising a sodium cation and a hypochlorite anion. It may also be viewed as the sodium salt of hypochlorous acid. The anhydrous compound is unstable and may decompose explosively. It can be crystallized as a pentahydrate NaOCl·5H
2O, a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated.

Chlorine dioxide is a chemical compound with the formula ClO2 that exists as yellowish-green gas above 11 °C, a reddish-brown liquid between −59 °C and 11 °C, and as bright orange crystals when colder. It is an oxidizing agent, able to transfer oxygen to a variety of substrates, while gaining one or more electrons via oxidation-reduction (redox). It does not hydrolyze when it enters water, and is usually handled as a dissolved gas in solution in water. Potential hazards with chlorine dioxide include health concerns, explosiveness and fire ignition. It is commonly used as a bleach.

In chemistry, neutralization or neutralisation is a chemical reaction in which an acid and a base react quantitatively with each other. In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution. The pH of the neutralized solution depends on the acid strength of the reactants.

Sodium acetate, CH3COONa, also abbreviated NaOAc, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.
Disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term can be applied to any desymmetrizing reaction of the following type: 2 A → A' + A", regardless of whether it is a redox or some other type of process.
Reaction in which two compounds in their aqueous solution react to form two new product by interchanging their radicals or ions is called a double decomposition reaction or double displacement reaction

The oxidation state of oxygen is −2 in almost all known compounds of oxygen. The oxidation state −1 is found in a few compounds such as peroxides. Compounds containing oxygen in other oxidation states are very uncommon: −1⁄2 (superoxides), −1⁄3 (ozonides), 0, +1⁄2 (dioxygenyl), +1, and +2.
McIntosh and Filde's anaerobic jar is an instrument used in the production of an anaerobic environment. This method of anaerobiosis as others is used to culture bacteria which die or fail to grow in presence of oxygen (anaerobes).

Sodium bisulfite (or sodium bisulphite, sodium hydrogen sulfite) is a chemical mixture with the approximate chemical formula NaHSO3. Sodium bisulfite in fact is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of sodium and bisulfite ions. It is a white solid with an odor of sulfur dioxide. Regardless of its ill-defined nature, "sodium bisulfite" is a food additive with E number E222.

Dry ice color show is the formation of carbonic acid (H2CO3) by the reaction of dry ice, the solid form of carbon dioxide (CO2), and water. The reaction between these 2 compounds lower the pH of the solution, therefore making the solution more acidic. Dry ice has a temperature of –78.5 °C (-109.3°F)and normally exist in gas state. By applying universal indicator to the solution, the changing of the pH can be indicated as the color of the solution changes into the contrast color. This experiment is usually conducted as a classroom demonstration of pH and properties of carbon dioxide since the materials required are handful and can be prepared easily. Solid carbon dioxide can cause frostbite when in contact with the skin, by sticking to the moist tissue, such as wet skin or tongue. Solid carbon dioxide undergoes sublimation upon exposure to air. This means it transforms directly from the solid phase to the gaseous phase without melting into liquid.














