
Novartis AG is a Swiss multinational pharmaceutical corporation based in Basel, Switzerland. Consistently ranked in the global top five, Novartis is one of the largest pharmaceutical companies in the world and was the fourth largest by revenue in 2022.
Genentech, Inc. is an American biotechnology corporation headquartered in South San Francisco, California. It became an independent subsidiary of Roche in 2009. Genentech Research and Early Development operates as an independent center within Roche. Historically, the company is regarded as the world's first biotechnology company.

Amgen Inc. is an American multinational biopharmaceutical company headquartered in Thousand Oaks, California. One of the world's largest independent biotechnology companies, As of 2022, Amgen has approximately 24,000 staff in total.

Celgene Corporation is a pharmaceutical company that makes cancer and immunology drugs. Its major product is Revlimid (lenalidomide), which is used in the treatment of multiple myeloma, and also in certain anemias. The company is incorporated in Delaware, headquartered in Summit, New Jersey, and a subsidiary of Bristol Myers Squibb (BMS).

Regeneron Pharmaceuticals, Inc. is an American biotechnology company headquartered in Westchester County, New York. The company was founded in 1988. Originally focused on neurotrophic factors and their regenerative capabilities, giving rise to its name, the company then branched out into the study of both cytokine and tyrosine kinase receptors, which gave rise to their first product, which is a VEGF-trap.

Daiichi Sankyo Company, Limited is a global pharmaceutical company and the second-largest pharmaceutical company in Japan. It achieved JPY 1,278 billion in revenue in 2022. The company owns the American pharmaceutical company American Regent.
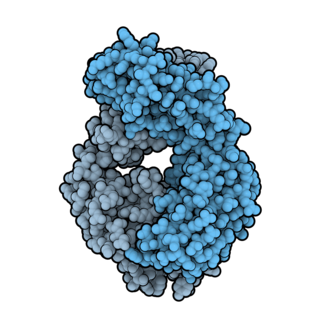
Ofatumumab is a fully human monoclonal antibody to CD20, which appears to provide rapid B-cell depletion. Under the brand name Kesimpta, it is approved for the treatment of multiple sclerosis in the United States as well as in the European Union and other regions. Under the brand name Arzerra, it is approved for the treatment of certain types of chronic lymphocytic leukemia (CLL) in the United States. It is sold by Novartis under license from Genmab.

Chugai Pharmaceutical Co., Ltd. is a drug manufacturer operating in Japan. It is a subsidiary controlled by Hoffmann-La Roche, which owns 62% of the company as of 30 June 2014. The company is headquartered in Tokyo. Osamu Nagayama is the current representative director and chairman. Tatsuro Kosaka is the current representative director, president and CEO.
MorphoSys AG is a German biopharmaceutical company founded in 1992. The company is headquartered near Munich, Germany, and has a wholly owned subsidiary, MorphoSys US Inc., in Boston, Massachusetts, in the US. The company has various antibody, protein and peptide technologies that it uses to discover and develop both proprietary and partnered drug candidates. The company has more than 100 drugs in its wider pipeline that are being investigated for a variety of diseases. While many of these are being developed in partnership with pharma and biotech companies, MorphoSys also has a proprietary pipeline with a focus on cancer and autoimmune diseases.

Merck & Co., Inc. is an American multinational pharmaceutical company headquartered in Rahway, New Jersey, and is named for Merck Group, founded in Germany in 1668, of which it was once the American arm. The company does business as Merck Sharp & Dohme or MSD outside the United States and Canada. It is one of the largest pharmaceutical companies in the world, generally ranking in the global top five by revenue.

Secukinumab, sold under the brand name Cosentyx among others, is a human IgG1κ monoclonal antibody used for the treatment of psoriasis, ankylosing spondylitis, and psoriatic arthritis. It binds to the protein interleukin (IL)-17A and is marketed by Novartis.

Daratumumab, sold under the brand name Darzalex among others, is an anti-cancer monoclonal antibody medication. It binds to CD38, which is overexpressed in multiple myeloma cells. Daratumumab was originally developed by Genmab, but it is now being jointly developed by Genmab along with the Johnson & Johnson subsidiary Janssen Biotech, which acquired worldwide commercialization rights to the drug from Genmab.
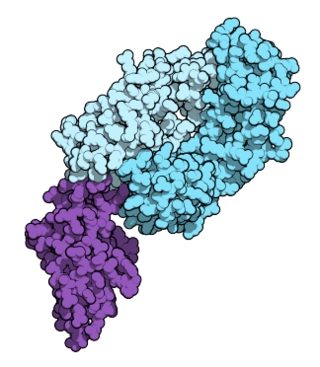
Nivolumab, sold under the brand name Opdivo, is an anti-cancer medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction cancer. It is administered intravenously.

Moderna, Inc. is an American pharmaceutical and biotechnology company based in Cambridge, Massachusetts, that focuses on RNA therapeutics, primarily mRNA vaccines. These vaccines use a copy of a molecule called messenger RNA (mRNA) to carry instructions for proteins to produce an immune response. The company's name is derived from the terms "modified", "RNA", and "modern".
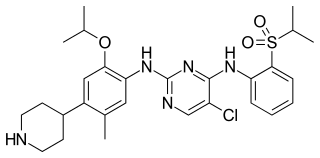
Ceritinib is a prescription-only drug used for the treatment of non-small cell lung cancer (NSCLC). It was developed by Novartis and received FDA approval for use in April 2014.
Dinutuximab and dinutuximab beta are monoclonal antibodies used as a second-line treatment for children with high-risk neuroblastoma. Each antibody is made of both mouse and human components and targets glycolipid GD2, expressed on neuroblastoma cells and on normal cells of neuroectodermal origin, including the central nervous system and peripheral nerves. They differ in that dinutuximab is manufactured using mouse cells, and dinutuximab beta is manufactured using hamster cells. The dosing regime differs, and dinutuximab is given in combination with granulocyte-macrophage colony stimulating factor (GM-CSF), interleukin-2 (IL-2) and 13-cis-retinoic acid (RA), while dinutuximab beta can be given alone.
Tisagenlecleucel, sold under the brand name Kymriah, is a CAR T cells medication for the treatment of B-cell acute lymphoblastic leukemia (ALL) which uses the body's own T cells to fight cancer.
Inclisiran, sold under the brand name Leqvio, is a medication used for the treatment of high low-density lipoprotein (LDL) cholesterol and for the treatment of people with atherosclerotic cardiovascular disease (ASCVD), ASCVD risk-equivalents, and heterozygous familial hypercholesterolemia (HeFH). It is a small interfering RNA (siRNA) that acts as an inhibitor of a proprotein convertase, specifically, inhibiting translation of the protein PCSK9.
AbCellera Biologics Inc. is a Vancouver, British Columbia-based biotechnology firm that researches and develops human antibodies. The company is best known for its leading role in the Pandemic Prevention Platform, a project of DARPA's Biological Technologies Office. AbCellera utilizes a proprietary technology platform, which they claim can develop "medical countermeasures within 60 days." Its platform for single-cell screening was initially developed at the University of British Columbia.
BeiGene, Ltd. is a China-based drug developer. It specializes in the development of drugs for cancer treatment. Founded in 2010 by chief executive officer John V. Oyler and Xiaodong Wang, the multinational company headquartered in Cambridge, Massachusetts has offices in North America, Europe, South America, Asia and Australia. BeiGene has a large presence in Chinese market. BeiGene has developed several pharmaceuticals, including tislelizumab, a checkpoint inhibitor, and zanubrutinib, a Bruton's tyrosine kinase inhibitor.













