Serine is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, a carboxyl group, and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC.

Leghemoglobin is an oxygen-carrying phytoglobin found in the nitrogen-fixing root nodules of leguminous plants. It is produced by these plants in response to the roots being colonized by nitrogen-fixing bacteria, termed rhizobia, as part of the symbiotic interaction between plant and bacterium: roots not colonized by Rhizobium do not synthesise leghemoglobin. Leghemoglobin has close chemical and structural similarities to hemoglobin, and, like hemoglobin, is red in colour. It was originally thought that the heme prosthetic group for plant leghemoglobin was provided by the bacterial symbiont within symbiotic root nodules. However, subsequent work shows that the plant host strongly expresses heme biosynthesis genes within nodules, and that activation of those genes correlates with leghemoglobin gene expression in developing nodules.
Saponins are bitter-tasting usually toxic plant-derived secondary metabolites, being organic chemicals, that have a foamy quality when agitated in water and a high molecular weight. They are present in a wide range of plant species throughout the bark, leaves, stems, roots and flowers but found particularly in soapwort, a flowering plant, the soapbark tree, common corn-cockle, baby's breath and soybeans. They are used in soaps, medicines, fire extinguishers, as dietary supplements, for synthesis of steroids, and in carbonated beverages. Saponins are both water and fat soluble, which gives them their useful soap properties. Some examples of these chemicals are glycyrrhizin and quillaia, a bark extract used in beverages.

Root nodules are found on the roots of plants, primarily legumes, that form a symbiosis with nitrogen-fixing bacteria. Under nitrogen-limiting conditions, capable plants form a symbiotic relationship with a host-specific strain of bacteria known as rhizobia. This process has evolved multiple times within the legumes, as well as in other species found within the Rosid clade. Legume crops include beans, peas, and soybeans.
Isoflavones are substituted derivatives of isoflavone, a type of naturally occurring isoflavonoids, many of which act as phytoestrogens in mammals. Isoflavones occur in many plant species, but are especially high in soybeans.

Fasciation, also known as cresting, is a relatively rare condition of abnormal growth in vascular plants in which the apical meristem, which normally is concentrated around a single point and produces approximately cylindrical tissue, instead becomes elongated perpendicularly to the direction of growth, thus producing flattened, ribbon-like, crested, or elaborately contorted tissue. Fasciation may also cause plant parts to increase in weight and volume in some instances. The phenomenon may occur in the stem, root, fruit, or flower head.

Daidzein is a naturally occurring compound found exclusively in soybeans and other legumes and structurally belongs to a class of compounds known as isoflavones. Daidzein and other isoflavones are produced in plants through the phenylpropanoid pathway of secondary metabolism and are used as signal carriers, and defense responses to pathogenic attacks. In humans, recent research has shown the viability of using daidzein in medicine for menopausal relief, osteoporosis, blood cholesterol, and lowering the risk of some hormone-related cancers, and heart disease. Despite the known health benefits, the use of both puerarin and daidzein is limited by their poor bioavailability and low water solubility.

Indoleamine-pyrrole 2,3-dioxygenase (IDO or INDO EC 1.13.11.52) is a heme-containing enzyme physiologically expressed in a number of tissues and cells, such as the small intestine, lungs, female genital tract or placenta. In humans is encoded by the IDO1 gene. IDO is involved in tryptophan metabolism. It is one of three enzymes that catalyze the first and rate-limiting step in the kynurenine pathway, the O2-dependent oxidation of L-tryptophan to N-formylkynurenine, the others being indolamine-2,3-dioxygenase 2 (IDO2) and tryptophan 2,3-dioxygenase (TDO). IDO is an important part of the immune system and plays a part in natural defense against various pathogens. It is produced by the cells in response to inflammation and has an immunosuppressive function because of its ability to limit T-cell function and engage mechanisms of immune tolerance. Emerging evidence suggests that IDO becomes activated during tumor development, helping malignant cells escape eradication by the immune system. Expression of IDO has been described in a number of types of cancer, such as acute myeloid leukemia, ovarian cancer or colorectal cancer. IDO is part of the malignant transformation process and plays a key role in suppressing the anti-tumor immune response in the body, so inhibiting it could increase the effect of chemotherapy as well as other immunotherapeutic protocols. Furthermore, there is data implicating a role for IDO1 in the modulation of vascular tone in conditions of inflammation via a novel pathway involving singlet oxygen.

Soybean mosaic virus (SMV) is a member of the plant virus genus Potyvirus. It infects mainly plants belonging to the family Fabaceae but has also been found infecting other economically important crops. SMV is the cause of soybean mosaic disease that occurs in all the soybean production areas of the world. Soybean is one of the most important sources of edible oil and proteins and pathogenic infections are responsible for annual yield losses of about $4 billion in the United States. Among these pathogens, SMV is the most important and prevalent viral pathogen in soybean production worldwide. It causes yield reductions of about 8% to 35%, but losses as high as 94% have been reported.
In enzymology, glyceollin synthase is an enzyme that catalyzes the last committed step in glyceollin biosynthesis. This enzyme has been classified as a cytochrome dependent monooxygenase. It uses cyclization of prenyl residue to convert glyceollidins into glyceollins.

The soybean aphid is an insect pest of soybean that is exotic to North America. The soybean aphid is native to Asia. It has been described as a common pest of soybeans in China and as an occasional pest of soybeans in Indonesia, Japan, Korea, Malaysia, the Philippines, and Thailand. The soybean aphid was first documented in North America in Wisconsin in July 2000. Ragsdale et al. (2004) noted that the soybean aphid probably arrived in North America earlier than 2000, but remained undetected for a period of time. Venette and Ragsdale (2004) suggested that Japan probably served as the point of origin for the soybean aphid's North American invasion. By 2003, the soybean aphid had been documented in Delaware, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New York, North Dakota, Ohio, Pennsylvania, South Dakota, Virginia, West Virginia, and Wisconsin. Together, these states accounted for 89% of the 63,600,000 acres (257,000 km2) of soybean planted in the United States in 2007.

Glycine soja, known as wild soybean, is an annual plant in the family Fabaceae. It may be treated as a separate species, the closest living relative of the cultivated soybean, Glycine max, an important crop, or as a subspecies of the cultivated soybean, Glycine max subsp. soja.
Bradyrhizobium japonicum is a species of legume-root nodulating, microsymbiotic nitrogen-fixing bacteria. The species is one of many Gram-negative, rod-shaped bacteria commonly referred to as rhizobia. Within that broad classification, which has three groups, taxonomy studies using DNA sequencing indicate that B. japonicum belongs within homology group II.

Pterocarpans are derivatives of isoflavonoids found in the family Fabaceae. It is a group of compounds which can be described as benzo-pyrano-furano-benzenes which can be formed by coupling of the B ring to the 4-one position.
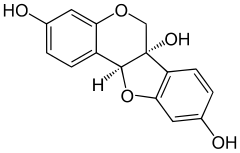
Glyceollins are a family of prenylated pterocarpans found in ineffective types of nodule in soybean in response to symbiotic infection.
Glyceollin I is a glyceollin, a type of prenylated pterocarpan. It is a phytoalexin found in the soybean.

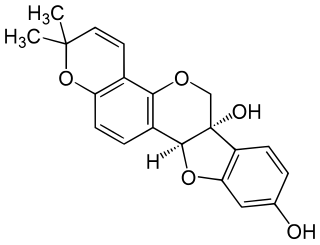
Glyceollin III is a glyceollin, a type of pterocarpan, found in the soybean. It has an antiestrogenic effect. In soil, it has an antifungal activity against Aspergillus sojae.

Aspergillus sojae is a species of fungus in the genus Aspergillus.

Gelsemine (C20H22N2O2) is an indole alkaloid isolated from flowering plants of the genus Gelsemium, a plant native to the subtropical and tropical Americas, and southeast Asia, and is a highly toxic compound that acts as a paralytic, exposure to which can result in death. It has generally potent activity as an agonist of the mammalian glycine receptor, the activation of which leads to an inhibitory postsynaptic potential in neurons following chloride ion influx, and systemically, to muscle relaxation of varying intensity and deleterious effect. Despite its danger and toxicity, recent pharmacological research has suggested that the biological activities of this compound may offer opportunities for developing treatments related to xenobiotic or diet-induced oxidative stress, and of anxiety and other conditions, with ongoing research including attempts to identify safer derivatives and analogs to make use of gelsemine's beneficial effects.
Ensifer fredii is a nitrogen fixing bacterium. It is a fast-growing root nodule bacterium. Ensifer fredii exhibits a broad host-range and is able to nodulate both determinant hosts, such as soy, as well as indeterminate hosts including the pigeon pea. Because of their ease of host infection there is interest in their genetics and the symbiotic role in host infection and nodule formation.