GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved P-loop "G domain", a protein domain common to many GTPases.

G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases.
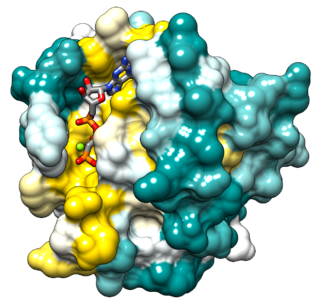
Ras, from "Rat sarcoma virus", is a family of related proteins that are expressed in all animal cell lineages and organs. All Ras protein family members belong to a class of protein called small GTPase, and are involved in transmitting signals within cells. Ras is the prototypical member of the Ras superfamily of proteins, which are all related in three-dimensional structure and regulate diverse cell behaviours.
Small GTPases, also known as small G-proteins, are a family of hydrolase enzymes that can bind and hydrolyze guanosine triphosphate (GTP). They are a type of G-protein found in the cytosol that are homologous to the alpha subunit of heterotrimeric G-proteins, but unlike the alpha subunit of G proteins, a small GTPase can function independently as a hydrolase enzyme to bind to and hydrolyze a guanosine triphosphate (GTP) to form guanosine diphosphate (GDP). The best-known members are the Ras GTPases and hence they are sometimes called Ras subfamily GTPases.
GTPase-activating proteins or GTPase-accelerating proteins (GAPs) are a family of regulatory proteins whose members can bind to activated G proteins and stimulate their GTPase activity, with the result of terminating the signaling event. GAPs are also known as RGS protein, or RGS proteins, and these proteins are crucial in controlling the activity of G proteins. Regulation of G proteins is important because these proteins are involved in a variety of important cellular processes. The large G proteins, for example, are involved in transduction of signaling from the G protein-coupled receptor for a variety of signaling processes like hormonal signaling, and small G proteins are involved in processes like cellular trafficking and cell cycling. GAP's role in this function is to turn the G protein's activity off. In this sense, GAPs function is opposite to that of guanine nucleotide exchange factors (GEFs), which serve to enhance G protein signaling.

Guanine nucleotide exchange factors (GEFs) are proteins or protein domains that activate monomeric GTPases by stimulating the release of guanosine diphosphate (GDP) to allow binding of guanosine triphosphate (GTP). A variety of unrelated structural domains have been shown to exhibit guanine nucleotide exchange activity. Some GEFs can activate multiple GTPases while others are specific to a single GTPase.

Heterotrimeric G protein, also sometimes referred to as the "large" G proteins are membrane-associated G proteins that form a heterotrimeric complex. The biggest non-structural difference between heterotrimeric and monomeric G protein is that heterotrimeric proteins bind to their cell-surface receptors, called G protein-coupled receptors, directly. These G proteins are made up of alpha (α), beta (β) and gamma (γ) subunits. The alpha subunit is attached to either a GTP or GDP, which serves as an on-off switch for the activation of G-protein.
G alpha subunits are one of the three types of subunit of guanine nucleotide binding proteins, which are membrane-associated, heterotrimeric G proteins.

Guanine nucleotide-binding protein G(q) subunit alpha is a protein that in humans is encoded by the GNAQ gene. Together with GNA11, it functions as a Gq alpha subunit.

Guanine nucleotide-binding protein G(i), alpha-1 subunit is a protein that in humans is encoded by the GNAI1 gene.

Regulators of G protein signaling (RGS) are protein structural domains or the proteins that contain these domains, that function to activate the GTPase activity of heterotrimeric G-protein α-subunits.

Guanine nucleotide-binding protein G(o) subunit alpha is a protein that in humans is encoded by the GNAO1 gene.

A-kinase anchor protein 13 is a protein that in humans is encoded by the AKAP13 gene. This protein is also called AKAP-Lbc because it encodes the lymphocyte blast crisis (Lbc) oncogene, and ARHGEF13/RhoGEF13 because it contains a guanine nucleotide exchange factor (GEF) domain for the RhoA small GTP-binding protein.

Rho guanine nucleotide exchange factor 1 is a protein that in humans is encoded by the ARHGEF1 gene. This protein is also called RhoGEF1 or p115-RhoGEF.

Rho guanine nucleotide exchange factor 12 is a protein that in humans is encoded by the ARHGEF12 gene. This protein is also called RhoGEF12 or Leukemia-associated Rho guanine nucleotide exchange factor (LARG).

Regulator of G-protein signaling 12 is a protein that in humans is encoded by the RGS12 gene.

Regulator of G-protein signaling 14 (RGS14) is a protein that in humans is encoded by the RGS14 gene.
Guanine nucleotide-binding protein subunit alpha-13 is a protein that in humans is encoded by the GNA13 gene.
Guanine nucleotide-binding protein G(k) subunit alpha is a protein that in humans is encoded by the GNAI3 gene.

Guanine nucleotide-binding protein subunit alpha-12 is a protein that in humans is encoded by the GNA12 gene.

















