
Streptococcus is a genus of gram-positive coccus or spherical bacteria that belongs to the family Streptococcaceae, within the order Lactobacillales, in the phylum Bacillota. Cell division in streptococci occurs along a single axis, so as they grow, they tend to form pairs or chains that may appear bent or twisted. This differs from staphylococci, which divide along multiple axes, thereby generating irregular, grape-like clusters of cells. Most streptococci are oxidase-negative and catalase-negative, and many are facultative anaerobes.

Enterococcus is a large genus of lactic acid bacteria of the phylum Bacillota. Enterococci are gram-positive cocci that often occur in pairs (diplococci) or short chains, and are difficult to distinguish from streptococci on physical characteristics alone. Two species are common commensal organisms in the intestines of humans: E. faecalis (90–95%) and E. faecium (5–10%). Rare clusters of infections occur with other species, including E. casseliflavus, E. gallinarum, and E. raffinosus.
Bloodstream infections (BSIs), septicemia which include bacteremias when the infections are bacterial and fungemias when the infections are fungal, are infections present in the blood. Blood is normally a sterile environment, so the detection of microbes in the blood is always abnormal. A bloodstream infection is different from sepsis, which is the host response to bacteria.
The HACEK organisms are a group of fastidious Gram-negative bacteria that are an unusual cause of infective endocarditis, which is an inflammation of the heart due to bacterial infection. HACEK is an abbreviation of the initials of the genera of this group of bacteria: Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, Kingella. The HACEK organisms are a normal part of the human microbiota, living in the oral-pharyngeal region.
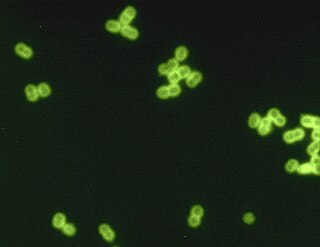
Streptococcus pneumoniae, or pneumococcus, is a Gram-positive, spherical bacteria, alpha-hemolytic member of the genus Streptococcus. They are usually found in pairs (diplococci) and do not form spores and are non motile. As a significant human pathogenic bacterium S. pneumoniae was recognized as a major cause of pneumonia in the late 19th century, and is the subject of many humoral immunity studies.

Infective endocarditis is an infection of the inner surface of the heart, usually the valves. Signs and symptoms may include fever, small areas of bleeding into the skin, heart murmur, feeling tired, and low red blood cell count. Complications may include backward blood flow in the heart, heart failure – the heart struggling to pump a sufficient amount of blood to meet the body's needs, abnormal electrical conduction in the heart, stroke, and kidney failure.

The viridans streptococci are a large group of commensal streptococcal Gram-positive bacteria species that are α-hemolytic, producing a green coloration on blood agar plates, although some species in this group are actually γ-hemolytic, meaning they produce no change on blood agar. The pseudo-taxonomic term "Streptococcus viridans" is often used to refer to this group of species, but writers who do not like to use the pseudotaxonomic term prefer the terms viridans streptococci, viridans group streptococci (VGS), or viridans streptococcal species.

Streptococcus mutans is a facultatively anaerobic, gram-positive coccus commonly found in the human oral cavity and is a significant contributor to tooth decay. It is part of the "streptococci", an informal general name for all species in the genus Streptococcus. The microbe was first described by James Kilian Clarke in 1924.

Subacute bacterial endocarditis, abbreviated SBE, is a type of endocarditis. Subacute bacterial endocarditis can be considered a form of type III hypersensitivity.
Streptococcus bovis is a species of Gram-positive bacteria that in humans is associated with urinary tract infections, endocarditis, sepsis, and colorectal cancer. S. gallolyticus is commonly found in the alimentary tract of cattle, sheep, and other ruminants, and may cause ruminal acidosis or feedlot bloat. It is also associated with spontaneous bacterial peritonitis, a frequent complication occurring in patients affected by cirrhosis. Equivalence with Streptococcus equinus has been contested.
Gemella morbillorum is a species of bacteria within the genus Gemella. It is a facultative anaerobic Gram positive coccus usually preferring capnophilic or microaerophilic environments. From its discovery in 1917 until 1988, it was known as Streptococcus morbillorum. The name change followed closer examination with DNA filter hybridization which found it was very close to the species Gemella haemolysans.
Streptococcus sanguinis, formerly known as Streptococcus sanguis, is a Gram-positive facultative anaerobic coccus species of bacteria and a member of the Viridans Streptococcus group. S. sanguinis is a normal inhabitant of the healthy human mouth where it is particularly found in dental plaque, where it modifies the environment to make it less hospitable for other strains of Streptococcus that cause cavities, such as Streptococcus mutans.

Arcanobacterium haemolyticum is a species of bacteria classified as a gram-positive bacillus. It is catalase-negative, facultative anaerobic, beta-hemolytic, and not motile. It has been known to cause head and neck infections, pharyngitis, and sinusitis.
Abiotrophia is a genus of lactic acid bacteria, a family in the phylum Bacillota (Bacteria).

The Streptococcus anginosus group (SAG), also known as the anginosus group streptococci (AGS) or the milleri group streptococci (MGS), are a group of several species of streptococci with clinical similarities. The group is named after a principal member species, Streptococcus anginosus. The older name Streptococcus milleri is now pseudotaxonomic, as the idea that these streptococci constituted a single species was incorrect. The anginosus group streptococci are members of the viridans streptococci group. They have been implicated as etiologic agents in a variety of serious purulent infections, but because of their heterogeneous characteristics, these organisms may be unrecognized or misidentified by clinical laboratorians. The unique characteristic of them from other pathogenic streptococci, such as S. pyogenes and S. agalactiae, is their ability to cause abscesses.
Streptococcus equinus is a Gram-positive, nonhemolytic, nonpathogenic, lactic acid bacterium of the genus Streptococcus. It is the principal Streptococcus found in the alimentary canal of a horse, and makes up the majority of the bacterial flora in horse feces. Equivalence with Streptococcus bovis has been contested.
Streptococcus vestibularis is a species of Streptococcus.
Dental antibiotic prophylaxis is the administration of antibiotics to a dental patient for prevention of harmful consequences of bacteremia, that may be caused by invasion of the oral flora into an injured gingival or peri-apical vessel during dental treatment.

Enzybiotics are an experimental antibacterial therapy first described by Nelson, Loomis, and Fischetti. The term is derived from a combination of the words “enzyme” and “antibiotics.” Enzymes have been extensively utilized for their antibacterial and antimicrobial properties. Proteolytic enzymes called endolysins have demonstrated particular effectiveness in combating a range of bacteria and are the basis for enzybiotic research. Endolysins are derived from bacteriophages and are highly efficient at lysing bacterial cells. Enzybiotics are being researched largely to address the issue of antibiotic resistance, which has allowed for the proliferation of drug-resistant pathogens posing great risk to animal and human health across the globe.
Streptococcus tigurinus is a novel member of the genus Streptococcus that was first discovered in 2012 by Swedish researchers.









