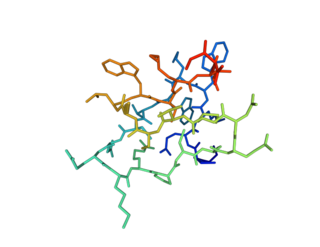
| M-theraphotoxin-Gr1a | |||||||
|---|---|---|---|---|---|---|---|
 | |||||||
| Identifiers | |||||||
| Organism | |||||||
| Symbol | GsMTx-4 | ||||||
| PDB | 1LU8 | ||||||
| UniProt | Q7YT39 | ||||||
| |||||||
| Names | |
|---|---|
| IUPAC name glycyl-cysteinyl-leucyl-alpha-glutamyl-phenylalanyl-tryptophyl-tryptophyl-lysyl-cysteinyl-asparagyl-prolyl-asparagyl-alpha-aspartyl-alpha-aspartyl-lysyl-cysteinyl-cysteinyl-arginyl-prolyl-lysyl-leucyl-lysyl-cysteinyl-seryl-lysyl-leucyl-phenylalanyl-lysyl-leucyl-cysteinyl-asparagyl-phenylalanyl-seryl-phenylalaninamide (2->17),(9->23),(16->30)-tris(disulfide) | |
Other names
| |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
PubChem CID | |
| |
| |
| Properties | |
| C185H273N49O45S6 | |
| Molar mass | 4095.88 g·mol−1 |
| 1 mg/mL | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Grammostola mechanotoxin #4 (GsMTx-4, GsMTx4, GsMTx-IV), also known as M-theraphotoxin-Gr1a (M-TRTX-Gr1a), is a neurotoxin isolated from the venom of the spider Chilean rose tarantula Grammostola spatulate (or Grammostola rosea ). [1] This amphiphilic peptide, which consists of 35 amino acids, belongs to the inhibitory cysteine knot (ICK) peptide family. [2] It reduces mechanical sensation by inhibiting mechanosensitive channels (MSCs). [3]
Contents
- Source
- Chemistry
- Structure
- Homology
- Properties
- Target
- Mode of action
- Binding affinity
- Therapeutic use
- References
GsMTx-4 also serves as a cationic antimicrobial peptide against Gram-positive bacteria. [4]