
Chlorine is a chemical element; it has symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Pauling scale, behind only oxygen and fluorine.

In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to an organyl group, or hydrogen, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

Polyvinyl chloride (alternatively: poly(vinyl chloride), colloquial: vinyl or polyvinyl; abbreviated: PVC) is the world's third-most widely produced synthetic polymer of plastic (after polyethylene and polypropylene). About 40 million tons of PVC are produced each year.
The term chloride refers to a compound or molecule that contains either a chlorine anion, which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond. Many inorganic chlorides are salts. Many organic compounds are chlorides. The pronunciation of the word "chloride" is.

Sodium chloride, commonly known as edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chlorine ions. It is transparent or translucent, brittle, hygroscopic, and occurs as the mineral halite. In its edible form, it is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is deicing of roadways in sub-freezing weather.

Potassium chloride is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water, and its solutions have a salt-like taste. Potassium chloride can be obtained from ancient dried lake deposits. KCl is used as a fertilizer, in medicine, in scientific applications, domestic water softeners, and in food processing, where it may be known as E number additive E508.
The compound hydrogen chloride has the chemical formula HCl and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. Hydrochloric acid, the aqueous solution of hydrogen chloride, is also commonly given the formula HCl.

Ammonium chloride is an inorganic chemical compound with the chemical formula NH4Cl, also written as [NH4]Cl. It is an ammonium salt of hydrogen chloride. It consists of ammonium cations [NH4]+ and chloride anions Cl−. It is a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. In its naturally occurring mineralogic form, it is known as salammoniac. The mineral is commonly formed on burning coal dumps from condensation of coal-derived gases. It is also found around some types of volcanic vents. It is mainly used as fertilizer and a flavouring agent in some types of liquorice. It is a product of the reaction of hydrochloric acid and ammonia.

Vinyl chloride is an organochloride with the formula H2C=CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. It is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC). Vinyl chloride is a colourless flammable gas that has a sweet odor and is carcinogenic. Vinyl chloride monomer is among the top twenty largest petrochemicals (petroleum-derived chemicals) in world production. The United States remains the largest vinyl chloride manufacturing region because of its low-production-cost position in chlorine and ethylene raw materials. China is also a large manufacturer and one of the largest consumers of vinyl chloride. It can be formed in the environment when soil organisms break down chlorinated solvents. Vinyl chloride that is released by industries or formed by the breakdown of other chlorinated chemicals can enter the air and drinking water supplies. Vinyl chloride is a common contaminant found near landfills. Before the 1970s, vinyl chloride was used as an aerosol propellant and refrigerant.

Calcium chloride is an inorganic compound, a salt with the chemical formula CaCl2. It is a white crystalline solid at room temperature, and it is highly soluble in water. It can be created by neutralising hydrochloric acid with calcium hydroxide.

Mercury(II) chloride (or mercury bichloride, mercury dichloride), historically also known as sulema or corrosive sublimate, is the inorganic chemical compound of mercury and chlorine with the formula HgCl2, used as a laboratory reagent. It is a white crystalline solid and a molecular compound that is very toxic to humans. Once used as a treatment for syphilis, it is no longer used for medicinal purposes because of mercury toxicity and the availability of superior treatments.
Iron(III) chloride describes the inorganic compounds with the formula FeCl3(H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms, which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agents. It is used as a water cleaner and as an etchant for metals.

Zinc chloride is an inorganic chemical compound with the formula ZnCl2·nH2O, with n ranging from 0 to 4.5, forming hydrates. Zinc chloride, anhydrous and its hydrates, are colorless or white crystalline solids, and are highly soluble in water. Five hydrates of zinc chloride are known, as well as four forms of anhydrous zinc chloride.
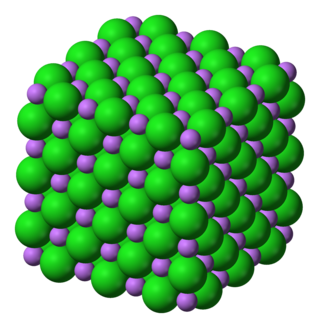
Lithium chloride is a chemical compound with the formula LiCl. The salt is a typical ionic compound (with certain covalent characteristics), although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorides, such as extraordinary solubility in polar solvents (83.05 g/100 mL of water at 20 °C) and its hygroscopic properties.

Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula AlCl3. It forms a hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour.

Chloride channels are a superfamily of poorly understood ion channels specific for chloride. These channels may conduct many different ions, but are named for chloride because its concentration in vivo is much higher than other anions. Several families of voltage-gated channels and ligand-gated channels have been characterized in humans.

Thionyl chloride is an inorganic compound with the chemical formula SOCl2. It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately 45,000 tonnes per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.

Rubidium chloride is the chemical compound with the formula RbCl. This alkali metal halide salt is composed of rubidium and chlorine, and finds diverse uses ranging from electrochemistry to molecular biology.

Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical.


















