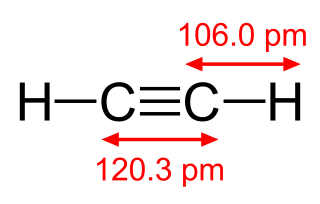
Acetylene is the chemical compound with the formula C2H2 and structure H−C≡C−H. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.
Isobutane, also known as i-butane, 2-methylpropane or methylpropane, is a chemical compound with molecular formula HC(CH3)3. It is an isomer of butane. Isobutane is a colorless, odorless gas. It is the simplest alkane with a tertiary carbon atom. Isobutane is used as a precursor molecule in the petrochemical industry, for example in the synthesis of isooctane.

1,4-Dichlorobenzene (1,4-DCB, p-DCB, or para-dichlorobenzene, sometimes abbreviated as PDCB or para) is an organic compound with the formula C6H4Cl2. This colorless solid has a strong odor. The molecule consists of a benzene ring with two chlorine atoms (replacing hydrogen atoms) on opposing sites of the ring.

Tetrachloroethylene, also known under the systematic name tetrachloroethene, or perchloroethylene, and abbreviations such as "perc" (or "PERC"), and "PCE", is a chlorocarbon with the formula Cl2C=CCl2. It is a colorless liquid widely used for dry cleaning of fabrics, hence it is sometimes called "dry-cleaning fluid". It also has its uses as an effective automotive brake cleaner. It has a mild sweet odor, similar to the smell of chloroform, detectable by most people at a concentration of 1 part per million (1 ppm).

The organic compound 1,1,1-trichloroethane, also known as methyl chloroform and chlorothene, is a chloroalkane with the chemical formula CH3CCl3. It is an isomer of 1,1,2-trichloroethane. This colorless, sweet-smelling liquid was once produced industrially in large quantities for use as a solvent. It is regulated by the Montreal Protocol as an ozone-depleting substance and its use is being rapidly phased out.
Organochlorine chemistry is concerned with the properties of organochlorine compounds, or organochlorides, organic compounds containing at least one covalently bonded atom of chlorine. The chloroalkane class includes common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious.

Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl chloride, which is used to make polyvinyl chloride (PVC) pipes, furniture and automobile upholstery, wall coverings, housewares, and automobile parts. 1,2-Dichloroethane is also used generally as an intermediate for other organic chemical compounds, and as a solvent. It forms azeotropes with many other solvents, including water and other chlorocarbons.
Camphene is a bicyclic organic compound. It is one of the most pervasive monoterpenes. As with other terpenes, it is insoluble in water, flammable, colorless, and has a pungent smell. It is a minor constituent of many essential oils such as turpentine, cypress oil, camphor oil, citronella oil, neroli, ginger oil, valerian, and mango. It is produced industrially by isomerization of the more common alpha-pinene using a solid acid catalyst such as titanium dioxide.
1,1-Dichloroethene, commonly called 1,1-dichloroethylene or vinylidene chloride or 1,1-DCE, is an organochloride with the molecular formula C2H2Cl2. It is a colorless liquid with a sharp odor. Like most chlorocarbons, it is poorly soluble in water, but soluble in organic solvents. 1,1-DCE was the precursor to the original clingwrap, Saran, for food, but this application has been phased out.
Antimony trifluoride is the inorganic compound with the formula SbF3. Sometimes called Swarts' reagent, it is one of two principal fluorides of antimony, the other being SbF5. It appears as a white solid. As well as some industrial applications, it is used as a reagent in inorganic and organofluorine chemistry.
1,2,4-Trichlorobenzene is an organochlorine compound, one of three isomers of trichlorobenzene. It is a derivative of benzene with three chloride substituents. It is a colorless liquid used as a solvent for a variety of compounds and materials.

Thiophosphoryl chloride is an inorganic compound with the chemical formula PSCl3. It is a colorless pungent smelling liquid that fumes in air. It is synthesized from phosphorus chloride and used to thiophosphorylate organic compounds, such as to produce insecticides.
Perfluoroethers are a class of organofluorine compound containing one or more ether functional group. In general these compounds are structurally analogous to the related hydrocarbon ethers, except for the distinctive properties of fluorocarbons.
The chloride process is used to separate titanium from its ores. The goal of the process is to win high purity titanium dioxide from ores such as ilmenite (FeTiO3) and rutile (TiO2). The strategy exploits the volatility of TiCl4, which is readily purified and converted to the dioxide. Millions of tons of TiO2 are produced annually by this process, mainly for use as white pigments. The chloride process has largely displaced the older sulfate process, which relies on hot sulfuric acid to extract iron and other impurities from ores..

1,2-Dichloropropane is an organic compound classified as a chlorocarbon. It is a colorless, flammable liquid with a sweet odor. it is obtained as a byproduct of the production of epichlorohydrin, which is produced on a large scale.

Extraction in chemistry is a separation process consisting of the separation of a substance from a matrix. The distribution of a solute between two phases is an equilibrium condition described by partition theory. This is based on exactly how the analyte moves from the initial solvent into the extracting solvent. The term washing may also be used to refer to an extraction in which impurities are extracted from the solvent containing the desired compound.

Perchloromethyl mercaptan is the organosulfur compound with the formula CCl3SCl. It is mainly used as an intermediate for the synthesis of dyes and fungicides (captan, folpet). It is a colorless oil, although commercial samples are yellowish. It is insoluble in water but soluble in organic solvents. It has a foul, unbearable, acrid odor. Perchloromethyl mercaptan is the original name. The systematic name is trichloromethanesulfenyl chloride, because the compound is a sulfenyl chloride, not a mercaptan.

o-Cymene is an organic compound classified as an aromatic hydrocarbon. Its structure consists of a benzene ring ortho-substituted with a methyl group and an isopropyl group. It is a flammable colorless liquid which is nearly insoluble in water but soluble in organic solvents.

m-Cymene is an organic compound classified as an aromatic hydrocarbon. Its structure consists of a benzene ring meta-substituted with a methyl group and an isopropyl group. It is a flammable colorless liquid which is nearly insoluble in water but soluble in organic solvents.















