A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them (phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a functional change of the target protein (substrate) by changing enzyme activity, cellular location, or association with other proteins. The human genome contains about 500 protein kinase genes and they constitute about 2% of all human genes. There are two main types of protein kinase, the great majority are serine/threonine kinases, which phosphorylate the hydroxyl groups of serines and threonines in their targets and the other are tyrosine kinases, although additional types exist. Protein kinases are also found in bacteria and plants. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction.

Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events, most commonly protein phosphorylation catalyzed by protein kinases, which ultimately results in a cellular response. Proteins responsible for detecting stimuli are generally termed receptors, although in some cases the term sensor is used. The changes elicited by ligand binding in a receptor give rise to a biochemical cascade, which is a chain of biochemical events known as a signaling pathway.

Caulobacter crescentus is a Gram-negative, oligotrophic bacterium widely distributed in fresh water lakes and streams. The taxon is more properly known as Caulobacter vibrioides.
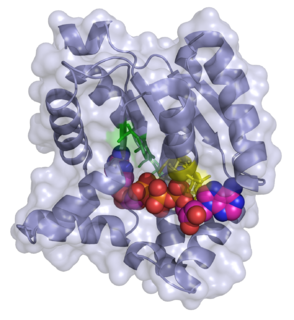
Adenylate kinase is a phosphotransferase enzyme that catalyzes the interconversion of the various adenosine phosphates. By constantly monitoring phosphate nucleotide levels inside the cell, ADK plays an important role in cellular energy homeostasis.
The IκB kinase (IKK) is an enzyme complex that is involved in propagating the cellular response to inflammation.

In enzymology, a protein-glutamate methylesterase (EC 3.1.1.61) is an enzyme that catalyzes the chemical reaction

In the field of molecular biology, a two-component regulatory system serves as a basic stimulus-response coupling mechanism to allow organisms to sense and respond to changes in many different environmental conditions. Two-component systems typically consist of a membrane-bound histidine kinase that senses a specific environmental stimulus and a corresponding response regulator that mediates the cellular response, mostly through differential expression of target genes. Although two-component signaling systems are found in all domains of life, they are most common by far in bacteria, particularly in Gram-negative and cyanobacteria; both histidine kinases and response regulators are among the largest gene families in bacteria. They are much less common in archaea and eukaryotes; although they do appear in yeasts, filamentous fungi, and slime molds, and are common in plants, two-component systems have been described as "conspicuously absent" from animals.

Histidine kinases (HK) are multifunctional, and in non-animal kingdoms, typically transmembrane, proteins of the transferase class of enzymes that play a role in signal transduction across the cellular membrane. The vast majority of HKs are homodimers that exhibit autokinase, phosphotransfer, and phosphatase activity. HKs can act as cellular receptors for signaling molecules in a way analogous to tyrosine kinase receptors (RTK). Multifunctional receptor molecules such as HKs and RTKs typically have portions on the outside of the cell that bind to hormone- or growth factor-like molecules, portions that span the cell membrane, and portions within the cell that contain the enzymatic activity. In addition to kinase activity, the intracellular domains typically have regions that bind to a secondary effector molecule or complex of molecules that further propagate signal transduction within the cell. Distinct from other classes of protein kinases, HKs are usually parts of a two-component signal transduction mechanisms in which HK transfers a phosphate group from ATP to a histidine residue within the kinase, and then to an aspartate residue on the receiver domain of a response regulator protein. More recently, the widespread existence of protein histidine phosphorylation distinct from that of two-component histidine kinases has been recognised in human cells. In marked contrast to Ser, Thr and Tyr phosphorylation, the analysis of phosphorylated Histidine using standard biochemical and mass spectrometric approaches is much more challenging, and special procedures and separation techniques are required for their preservation alongside classical Ser, Thr and Tyr phosphorylation on proteins isolated from human cells.

Protein phosphorylation is a reversible post-translational modification of proteins in which an amino acid residue is phosphorylated by a protein kinase by the addition of a covalently bound phosphate group. Phosphorylation alters the structural conformation of a protein, causing it to become activated, deactivated, or modifying its function. Approximately 13000 human proteins have sites that are phosphorylated.

Cell surface receptors are receptors that are embedded in the plasma membrane of cells. They act in cell signaling by receiving extracellular molecules. They are specialized integral membrane proteins that allow communication between the cell and the extracellular space. The extracellular molecules may be hormones, neurotransmitters, cytokines, growth factors, cell adhesion molecules, or nutrients; they react with the receptor to induce changes in the metabolism and activity of a cell. In the process of signal transduction, ligand binding affects a cascading chemical change through the cell membrane.
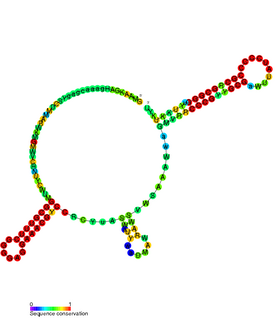
CrfA RNA is a family of non-coding RNAs found in Caulobacter crescentus. CrfA is expressed upon carbon starvation and is thought to activate 27 genes. It was originally identified along with 26 other non-coding RNAs using a tiled Caulobacter microarray protocol specifically aimed at detecting small RNAs.
The Methyl-accepting chemotaxis proteins are a family of transmembrane receptors that mediate chemotactic response in certain enteric bacteria, such as Salmonella typhimurium and Escherichia coli. These methyl-accepting chemotaxis receptors are one of the first components in the sensory excitation and adaptation responses in bacteria, which act to alter swimming behaviour upon detection of specific chemicals. Use of the MCP allows bacteria to detect concentrations of molecules in the extracellular matrix so that the bacteria may smooth swim or tumble accordingly. If the bacterium detects rising levels of attractants (nutrients) or declining levels of repellents (toxins), the bacterium will continue swimming forward, or smooth swimming. If the bacterium detects declining levels of attractants or rising levels of repellents, the bacterium will tumble and re-orient itself in a new direction. In this manner, a bacterium may swim towards nutrients and away from toxins
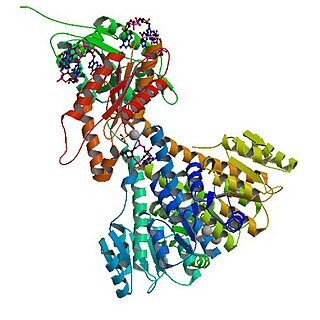
In enzymology, diguanylate cyclase, also known as diguanylate kinase, is an enzyme that catalyzes the chemical reaction:

In molecular biology, the protein domain Sda is short for suppressor of dnaA or otherwise known as sporulation inhibitor A. It is found only in bacteria. This protein domain is highly important to cell survival. When starved of nutrients, the cell is under extreme stress so undergoes a series of reactions to increase the chances of survival. One method is to form endospores which can withstand a large amount of environmental pressure. Sda protein domain is a checkpoint which prevents the formation of spores. The Sda domain affects cell signalling. It prevents the cell communicating the stress that it is under, which is crucial if the cell is to survive.
EnvZ/OmpR is a two-component regulatory system widely distributed in bacteria and particularly well characterized in Escherichia coli. Its function is in osmoregulation, responding to changes in environmental osmolality by regulating the expression of the outer membrane porins OmpF and OmpC. EnvZ is a histidine kinase which also possesses a cytoplasmic osmosensory domain, and OmpR is its corresponding response regulator protein.

A response regulator is a protein that mediates a cell's response to changes in its environment as part of a two-component regulatory system. Response regulators are coupled to specific histidine kinases which serve as sensors of environmental changes. Response regulators and histidine kinases are two of the most common gene families in bacteria, where two-component signaling systems are very common; they also appear much more rarely in the genomes of some archaea, yeasts, filamentous fungi, and plants. Two-component systems are not found in metazoans.
In molecular biology, the HAMP domain is an approximately 50-amino acid alpha-helical region that forms a dimeric, four-helical coiled coil. It is found in bacterial sensor and chemotaxis proteins and in eukaryotic histidine kinases. The bacterial proteins are usually integral membrane proteins and part of a two-component signal transduction pathway. One or several copies of the HAMP domain can be found in association with other domains, such as the histidine kinase domain, the bacterial chemotaxis sensory transducer domain, the PAS repeat, the EAL domain, the GGDEF domain, the protein phosphatase 2C-like domain, the guanylate cyclase domain, or the response regulatory domain. In its most common setting, the HAMP domain transmits conformational changes in periplasmic ligand-binding domains to cytoplasmic signalling kinase and methyl-acceptor domains and thus regulates the phosphorylation or methylation activity of homodimeric receptors.
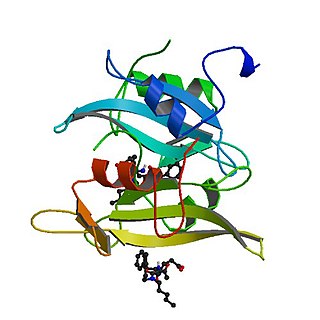
Tyrosine-protein kinase CSK also known as C-terminal Src kinase is an enzyme that, in humans, is encoded by the CSK gene. This enzyme phosphorylates tyrosine residues located in the C-terminal end of Src-family kinases (SFKs) including SRC, HCK, FYN, LCK, LYN and YES1.
The interleukin-1 receptor (IL-1R) associated kinase (IRAK) family plays a crucial role in the protective response to pathogens introduced into the human body by inducing acute inflammation followed by additional adaptive immune responses. IRAKs are essential components of the Interleukin-1 receptor signaling pathway and some Toll-like receptor signaling pathways. Toll-like receptors (TLRs) detect microorganisms by recognizing specific pathogen-associated molecular patterns (PAMPs) and IL-1R family members respond the interleukin-1 (IL-1) family cytokines. These receptors initiate an intracellular signaling cascade through adaptor proteins, primarily, MyD88. This is followed by the activation of IRAKs. TLRs and IL-1R members have a highly conserved amino acid sequence in their cytoplasmic domain called the Toll/Interleukin-1 (TIR) domain. The elicitation of different TLRs/IL-1Rs results in similar signaling cascades due to their homologous TIR motif leading to the activation of mitogen-activated protein kinases (MAPKs) and the IκB kinase (IKK) complex, which initiates a nuclear factor-κB (NF-κB) and AP-1-dependent transcriptional response of pro-inflammatory genes. Understanding the key players and their roles in the TLR/IL-1R pathway is important because the presence of mutations causing the abnormal regulation of Toll/IL-1R signaling leading to a variety of acute inflammatory and autoimmune diseases.
A cytokinin signaling and response regulator protein is a plant protein that is involved in a two step cytokinin signaling and response regulation pathway.