
The ozone layer or ozone shield is a region of Earth's stratosphere that absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the atmosphere, although still small in relation to other gases in the stratosphere. The ozone layer contains less than 10 parts per million of ozone, while the average ozone concentration in Earth's atmosphere as a whole is about 0.3 parts per million. The ozone layer is mainly found in the lower portion of the stratosphere, from approximately 15 to 35 kilometers (9 to 22 mi) above Earth, although its thickness varies seasonally and geographically.

The troposphere is the lowest layer of the atmosphere of Earth. It contains 80% of the total mass of the planetary atmosphere and 99% of the total mass of water vapor and aerosols, and is where most weather phenomena occur. From the planetary surface of the Earth, the average height of the troposphere is 18 km in the tropics; 17 km in the middle latitudes; and 6 km in the high latitudes of the polar regions in winter; thus the average height of the troposphere is 13 km.

Satellite temperature measurements are inferences of the temperature of the atmosphere at various altitudes as well as sea and land surface temperatures obtained from radiometric measurements by satellites. These measurements can be used to locate weather fronts, monitor the El Niño-Southern Oscillation, determine the strength of tropical cyclones, study urban heat islands and monitor the global climate. Wildfires, volcanos, and industrial hot spots can also be found via thermal imaging from weather satellites.

Ozone depletion consists of two related events observed since the late 1970s: a steady lowering of about four percent in the total amount of ozone in Earth's atmosphere, and a much larger springtime decrease in stratospheric ozone around Earth's polar regions. The latter phenomenon is referred to as the ozone hole. There are also springtime polar tropospheric ozone depletion events in addition to these stratospheric events.

The stratosphere is the second-lowest layer of the atmosphere of Earth, located above the troposphere and below the mesosphere. The stratosphere is composed of stratified temperature zones, with the warmer layers of air located higher and the cooler layers lower. The increase of temperature with altitude is a result of the absorption of the Sun's ultraviolet (UV) radiation by the ozone layer, where ozone is exothermically photolyzed into oxygen in a cyclical fashion. This temperature inversion is in contrast to the troposphere, where temperature decreases with altitude, and between the troposphere and stratosphere is the tropopause border that demarcates the beginning of the temperature inversion.

The mesosphere is the third layer of the atmosphere, directly above the stratosphere and directly below the thermosphere. In the mesosphere, temperature decreases as altitude increases. This characteristic is used to define limits: it begins at the top of the stratosphere, and ends at the mesopause, which is the coldest part of Earth's atmosphere, with temperatures below −143 °C. The exact upper and lower boundaries of the mesosphere vary with latitude and with season, but the lower boundary is usually located at altitudes from 47 to 51 km above sea level, and the upper boundary is usually from 85 to 100 km.

The thermosphere is the layer in the Earth's atmosphere directly above the mesosphere and below the exosphere. Within this layer of the atmosphere, ultraviolet radiation causes photoionization/photodissociation of molecules, creating ions; the thermosphere thus constitutes the larger part of the ionosphere. Taking its name from the Greek θερμός meaning heat, the thermosphere begins at about 80 km (50 mi) above sea level. At these high altitudes, the residual atmospheric gases sort into strata according to molecular mass. Thermospheric temperatures increase with altitude due to absorption of highly energetic solar radiation. Temperatures are highly dependent on solar activity, and can rise to 2,000 °C (3,630 °F) or more. Radiation causes the atmospheric particles in this layer to become electrically charged, enabling radio waves to be refracted and thus be received beyond the horizon. In the exosphere, beginning at about 600 km (375 mi) above sea level, the atmosphere turns into space, although, by the judging criteria set for the definition of the Kármán line (100 km), most of the thermosphere is part of space. The border between the thermosphere and exosphere is known as the thermopause.

The tropopause is the atmospheric boundary that demarcates the troposphere from the stratosphere, which are the lowest two of the five layers of the atmosphere of Earth. The tropopause is a thermodynamic gradient-stratification layer that marks the end of the troposphere, and is approximately 17 kilometres (11 mi) above the equatorial regions, and approximately 9 kilometres (5.6 mi) above the polar regions.

The atmosphere of Earth is composed of a layer of gas mixture that surrounds the Earth's planetary surface, known collectively as air, with variable quantities of suspended aerosols and particulates, all retained by Earth's gravity. The atmosphere serves as a protective buffer between the Earth's surface and outer space, shields the surface from most meteoroids and ultraviolet solar radiation, keeps it warm and reduces diurnal temperature variation through heat retention, redistributes heat and moisture among different regions via air currents, and provides the chemical and climate conditions allowing life to exist and evolve on Earth.

An atmosphere is a layer of gasses that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosphere is the outer region of a star, which includes the layers above the opaque photosphere; stars of low temperature might have outer atmospheres containing compound molecules.

Ground-level ozone (O3), also known as surface-level ozone and tropospheric ozone, is a trace gas in the troposphere (the lowest level of the Earth's atmosphere), with an average concentration of 20–30 parts per billion by volume (ppbv), with close to 100 ppbv in polluted areas. Ozone is also an important constituent of the stratosphere, where the ozone layer (2 to 8 parts per million ozone) exists which is located between 10 and 50 kilometers above the Earth's surface. The troposphere extends from the ground up to a variable height of approximately 14 kilometers above sea level. Ozone is least concentrated in the ground layer (or planetary boundary layer) of the troposphere. Ground-level or tropospheric ozone is created by chemical reactions between NOx gases (oxides of nitrogen produced by combustion) and volatile organic compounds (VOCs). The combination of these chemicals in the presence of sunlight form ozone. Its concentration increases as height above sea level increases, with a maximum concentration at the tropopause. About 90% of total ozone in the atmosphere is in the stratosphere, and 10% is in the troposphere. Although tropospheric ozone is less concentrated than stratospheric ozone, it is of concern because of its health effects. Ozone in the troposphere is considered a greenhouse gas, and may contribute to global warming.

The natural environment or natural world encompasses all biotic and abiotic things occurring naturally, meaning in this case not artificial. The term is most often applied to Earth or some parts of Earth. This environment encompasses the interaction of all living species, climate, weather and natural resources that affect human survival and economic activity. The concept of the natural environment can be distinguished as components:

The ozone–oxygen cycle is the process by which ozone is continually regenerated in Earth's stratosphere, converting ultraviolet radiation (UV) into heat. In 1930 Sydney Chapman resolved the chemistry involved. The process is commonly called the Chapman cycle by atmospheric scientists.

The TIMED mission is dedicated to study the influences energetics and dynamics of the Sun and humans on the least explored and understood region of Earth's atmosphere – the Mesosphere and Lower Thermosphere / Ionosphere (MLTI). The mission was launched from Vandenberg Air Force Base in California on 7 December 2001 aboard a Delta II rocket launch vehicle. The project is sponsored and managed by NASA, while the spacecraft was designed and assembled by the Applied Physics Laboratory at Johns Hopkins University. The mission has been extended several times, and has now collected data over an entire solar cycle, which helps in its goal to differentiate the Sun's effects on the atmosphere from other effects. It shared its Delta II launch vehicle with the Jason-1 oceanography mission.
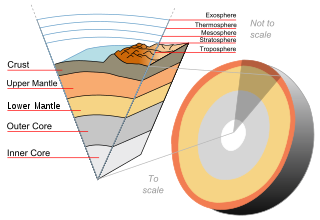
The following outline is provided as an overview of and topical guide to Earth science:
The turbopause, also called the homopause, marks the altitude in an atmosphere below which turbulent mixing dominates. Mathematically, it is defined as the point where the coefficient of Eddy diffusion is equal to the coefficient of molecular diffusion. The region below the turbopause is known as the homosphere, where the atmosphere is well mixed for chemical species which have long mean residence times. Highly reactive chemicals tend to have variable concentration throughout the atmosphere, while unreactive species have more homogeneous concentrations. The region above the turbopause is the heterosphere, where molecular diffusion dominates and the chemical composition of the atmosphere varies according to chemical species and their atomic weight.

The atmosphere of Venus is the very dense layer of gasses surrounding the planet Venus. Venus's atmosphere is composed of 96.5% carbon dioxide and 3.5% nitrogen, with other chemical compounds present only in trace amounts. It is much denser and hotter than that of Earth; the temperature at the surface is 740 K, and the pressure is 93 bar (1,350 psi), roughly the pressure found 900 m (3,000 ft) under water on Earth. The atmosphere of Venus supports decks of opaque clouds of sulfuric acid that cover the entire planet, preventing optical Earth-based and orbital observation of the surface. Information about surface topography has been obtained exclusively by radar imaging.

Over the last two centuries many environmental chemical observations have been made from a variety of ground-based, airborne, and orbital platforms and deposited in databases. Many of these databases are publicly available. All of the instruments mentioned in this article give online public access to their data. These observations are critical in developing our understanding of the Earth's atmosphere and issues such as climate change, ozone depletion and air quality. Some of the external links provide repositories of many of these datasets in one place. For example, the Cambridge Atmospheric Chemical Database, is a large database in a uniform ASCII format. Each observation is augmented with the meteorological conditions such as the temperature, potential temperature, geopotential height, and equivalent PV latitude.
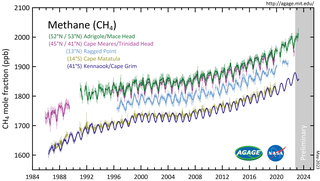
Atmospheric methane is the methane present in Earth's atmosphere. The concentration of atmospheric methane is increasing due to methane emissions, and is causing climate change. Methane is one of the most potent greenhouse gases. Methane's radiative forcing (RF) of climate is direct, and it is the second largest contributor to human-caused climate forcing in the historical period. Methane is a major source of water vapour in the stratosphere through oxidation; and water vapour adds about 15% to methane's radiative forcing effect. The global warming potential (GWP) for methane is about 84 in terms of its impact over a 20-year timeframe, and 28 in terms of its impact over a 100-year timeframe.

Atmospheric temperature is a measure of temperature at different levels of the Earth's atmosphere. It is governed by many factors, including incoming solar radiation, humidity, and altitude. The abbreviation MAAT is often used for Mean Annual Air Temperature of a geographical location.

















