
Limestone is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of CaCO3. Limestone forms when these minerals precipitate out of water containing dissolved calcium. This can take place through both biological and nonbiological processes, though biological processes, such as the accumulation of corals and shells in the sea, have likely been more important for the last 540 million years. Limestone often contains fossils which provide scientists with information on ancient environments and on the evolution of life.

Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses.

Dolomite is an anhydrous carbonate mineral composed of calcium magnesium carbonate, ideally CaMg(CO3)2. The term is also used for a sedimentary carbonate rock composed mostly of the mineral dolomite (see Dolomite (rock)). An alternative name sometimes used for the dolomitic rock type is dolostone.

Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate, the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation from marine and freshwater environments.
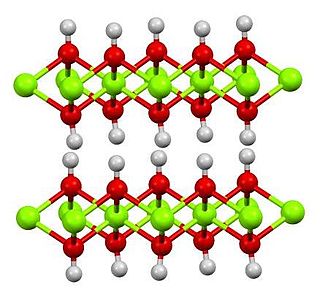
Magnesium hydroxide is an inorganic compound with the chemical formula Mg(OH)2. It occurs in nature as the mineral brucite. It is a white solid with low solubility in water (Ksp = 5.61×10−12). Magnesium hydroxide is a common component of antacids, such as milk of magnesia.

Brucite is the mineral form of magnesium hydroxide, with the chemical formula Mg(OH)2. It is a common alteration product of periclase in marble; a low-temperature hydrothermal vein mineral in metamorphosed limestones and chlorite schists; and formed during serpentinization of dunites. Brucite is often found in association with serpentine, calcite, aragonite, dolomite, magnesite, hydromagnesite, artinite, talc and chrysotile.

Magnesium carbonate, MgCO3, is an inorganic salt that is a colourless or white solid. Several hydrated and basic forms of magnesium carbonate also exist as minerals.

A speleothem is a geological formation made by mineral deposits that accumulate over time in natural caves. Speleothems most commonly form in calcareous caves due to carbonate dissolution reactions. They can take a variety of forms, depending on their depositional history and environment. Their chemical composition, gradual growth, and preservation in caves make them useful paleoclimatic proxies.

Magnesite is a mineral with the chemical formula MgCO
3. Iron, manganese, cobalt, and nickel may occur as admixtures, but only in small amounts.

Dolomite (also known as dolomite rock, dolostone or dolomitic rock) is a sedimentary carbonate rock that contains a high percentage of the mineral dolomite, CaMg(CO3)2. It occurs widely, often in association with limestone and evaporites, though it is less abundant than limestone and rare in Cenozoic rock beds (beds less than about 66 million years in age). One of the first geologists to distinguish dolomite from limestone was Déodat Gratet de Dolomieu, a French mineralogist and geologist after whom it is named. He recognized and described the distinct characteristics of dolomite in the late 18th century, differentiating it from limestone.

Artinite is a hydrated basic magnesium carbonate mineral with formula: Mg2(CO3)(OH)2·3H2O. It forms white silky monoclinic prismatic crystals that are often in radial arrays or encrustations. It has a Mohs hardness of 2.5 and a specific gravity of 2.

Barytocalcite is an anhydrous barium calcium carbonate mineral with the chemical formula BaCa(CO3)2. It is trimorphous with alstonite and paralstonite, that is to say the three minerals have the same formula but different structures. Baryte and quartz pseudomorphs after barytocalcite have been observed.

Carbonate rocks are a class of sedimentary rocks composed primarily of carbonate minerals. The two major types are limestone, which is composed of calcite or aragonite (different crystal forms of CaCO3), and dolomite rock (also known as dolostone), which is composed of dolomite (CaMg(CO3)2). They are usually classified on the basis of texture and grain size. Importantly, carbonate rocks can exist as metamorphic and igneous rocks, too. When recrystallized carbonate rocks are metamorphosed, marble is created. Rare igneous carbonate rocks even exist as intrusive carbonatites and, even rarer, there exists volcanic carbonate lava.

Hydromagnesite is a hydrated magnesium carbonate mineral with the formula Mg5(CO3)4(OH)2·4H2O.

Carbonate minerals are those minerals containing the carbonate ion, CO2−
3.

Anthodites (Greek ἄνθος ánthos, "flower", -ode, adjectival combining form, -ite adjectival suffix) are speleothems (cave formations) composed of long needle-like crystals situated in clusters which radiate outward from a common base. The "needles" may be quill-like or feathery. Most anthodites are made of the mineral aragonite (a variety of calcium carbonate, CaCO3), although some are composed of gypsum (CaSO4·2H2O).

Kutnohorite is a rare calcium manganese carbonate mineral with magnesium and iron that is a member of the dolomite group. It forms a series with dolomite, and with ankerite. The end member formula is CaMn2+(CO3)2, but Mg2+ and Fe2+ commonly substitute for Mn2+, with the manganese content varying from 38% to 84%, so the formula Ca(Mn2+,Mg,Fe2+)(CO3)2 better represents the species. It was named by Professor Bukowsky in 1901 after the type locality of Kutná Hora, Bohemia, in the Czech Republic. It was originally spelt "kutnahorite" but "kutnohorite" is the current IMA-approved spelling.

Monohydrocalcite is a mineral that is a hydrous form of calcium carbonate, CaCO3·H2O. It was formerly also known by the name hydrocalcite, which is now discredited by the IMA. It is a trigonal mineral which is white when pure. Monohydrocalcite is not a common rock-forming mineral, but is frequently associated with other calcium and magnesium carbonate minerals, such as calcite, aragonite, lansfordite, and nesquehonite.

Benstonite is a mineral with formula Ba6Ca6Mg(CO3)13. Discovered in 1954, the mineral was described in 1961 and named after Orlando J. Benston (1901–1966).

Mesitite (from Ancient Greek: μεσιτης — middle, between), formerly better known as mesitine spar, ferrous magnesite or brown spar is a variety of magnesite, a carbonate mineral, one of the so-called brown spars, regarded as an iron-bearing variety of magnesite or, in other cases, breunnerite. True to its name, mesitite is the middle member of a continuous isomorphic series magnesite—siderite with the general formula (Mg,Fe)CO3, in which the iron ion content (Fe2+) is approximately 30 to 50%, and the iron to magnesium ratio ranges, accordingly, from 30:70 to 50:50.




















